-
PDF
- Split View
-
Views
-
Cite
Cite
Mark Heffernan, Roger J. Summers, Anne Thorburn, Esra Ogru, Robert Gianello, Woei-Jia Jiang, Frank M. Ng, The Effects of Human GH and Its Lipolytic Fragment (AOD9604) on Lipid Metabolism Following Chronic Treatment in Obese Mice andβ 3-AR Knock-Out Mice, Endocrinology, Volume 142, Issue 12, 1 December 2001, Pages 5182–5189, https://doi.org/10.1210/endo.142.12.8522
Close - Share Icon Share
Abstract
Both human GH (hGH) and a lipolytic fragment (AOD9604) synthesized from its C-terminus are capable of inducing weight loss and increasing lipolytic sensitivity following long-term treatment in mice. One mechanism by which this may occur is through an interaction with theβ -adrenergic pathway, particularly with theβ 3-adrenergic receptors (β3-AR). Here we describe how hGH and AOD9604 can reduce body weight and body fat in obese mice following 14 d of chronic ip administration. These results correlate with increases in the level of expression ofβ 3-AR RNA, the major lipolytic receptor found in fat cells. Importantly, both hGH and AOD9604 are capable of increasing the repressed levels of β3-AR RNA in obese mice to levels comparable with those in lean mice. The importance ofβ 3-AR was verified when long-term treatment with hGH and AOD9604 in β3-AR knock-out mice failed to produce the change in body weight and increase in lipolysis that was observed in wild-type control mice. However, in an acute experiment, AOD9604 was capable of increasing energy expenditure and fat oxidation in theβ 3-AR knock-out mice. In conclusion, this study demonstrates that the lipolytic actions of both hGH and AOD9604 are not mediated directly through the β3-AR although both compounds increase β3-AR expression, which may subsequently contribute to enhanced lipolytic sensitivity.
HUMAN GH (hGH) has profound lipolytic/antilipogenic actions in vivo, which result in decreased fat mass, increased lean mass, and weight loss (1–3). Studies in vitro and in vivo have indicated that this response is mediated in part by an increase inβ -adrenoceptor coupling efficiency (4), a reduction in Gi expression (5), increased activity of hormone sensitive lipase (6), and an inhibitory effect on the action of insulin (7). We have synthesized a fragment of hGH (AOD9604) that contains a lipolytic domain that may be responsible for the lipolytic action of hGH. The parent molecule, AOD9401, induces lipolysis and antilipogenesis and fat oxidation in adipose tissue in vitro (8, 9). In vivo, AOD9401 induces weight loss without affecting food intake as well as increasing lipolytic sensitivity and increasing fat oxidation with no adverse effects on insulin sensitivity (8, 10).
The nature of the response to both hGH and AOD9604 is not clearly understood. We hypothesized that both molecules may influence the expression of the β3-adrenergic receptors (β3-ARs), the major lipolytic receptor in fat tissue. Both AOD9604 and hGH can increase β3-AR mRNA expression, as well as protein levels and function, in mouse and human cell lines in vitro (11). This response was investigated at the level of RNA and protein expression and function. The results for each mode of analysis were consistent in that both hGH and AOD9604 acted in a dose- and time-dependent manner to modulate the β3-AR response.
In this paper, we investigated whether the changes observed inβ 3-AR RNA expression in vitro also occur in an in vivo model. The in vivo model used was the obese (ob/ob) mouse model of obesity that has repressed levels of β3-ARs, which in part contributes to reduced lipolytic sensitivity (12). Lean C57BL/6J mice were used as a control. Following a 14-d chronic administration with AOD9604 or hGH, adipose tissue weights were measured, and β3-AR mRNA expression was determined. The decrease in weight of adipose tissue depots in the ob/ob mice was associated with increasedβ 3-AR expression. Further studies inβ 3-AR knock-out (β3-KO) mice showed that the presence of the β3-AR is necessary to mediate the chronic effectiveness of hGH and AOD9604 with regards to weight loss and fat mass reduction. However, an acute dose of AOD9604 was capable of increasing energy expenditure inβ 3-KO mice, although the response was less than that seen in the wild-type control mice.
Materials and Methods
Compounds
AOD9604, a synthetic fragment of hGH consisting of the amino acid residues 177–191 with the addition of a tyrosine residue to the N-terminus was prepared with solid-phase synthesis procedure and purified with reverse-phase HPLC methodology in our laboratories (13). The structure of the peptide analog was verified with mass spectrometry and amino acid analysis. The hGH was a gift of Professor Michael Waters (University of Queensland, Brisbane, Australia). BRL37344 (β3-AR agonist) was a gift from Dr. Jon Arch (SmithKline Beecham, Harlow, UK).
Animals and treatment
Lean C57BL/6J and obese (ob/ob) mice aged 12 wk were used in this study. There were 18 mice in each group, and they were divided into three treatment groups [saline (n = 6); AOD (250μ g/kg·d; n = 6); hGH (1 mg/k·d; n = 6)]. The animals were housed in the Departmental Animal Facility at a constant temperature and humidity in a 12-h light, 12-h dark cycle. Animals were injected with a single intraperitoneal dose of saline, AOD9604, or hGH at 0800 h each day for 14 d using a 1-ml syringe and 23-gauge needle. The body weights of the animals were recorded every second day along with food intake.
After 14 d, the animals were killed with 35 mg/kg sodium pentobarbitone (Rhone Merieux, Pinkenba, Queensland, Australia) injected into the heart. Their adipose tissues (white epididymal and brown interscapular) were removed and weighed. The tissues were frozen in liquid nitrogen and stored at −80 C until RNA analysis was performed.
The male β3-KO mice and wild-type (WT) that were used in this study were offspring of animals provided by Dr. Bradford Howell (Beth Israel Hospital, Harvard Medical School, Boston, MA). The animals were bred and housed in the central animal house facility (Monash University). For chronic studies, animals were housed in the Departmental Animal Facility (Biochemistry, Monash University). For acute energy expenditure studies, animals were transported to the Department of Medicine (University of Melbourne), acclimatized, and killed following experimentation. The WT andβ 3-KO mice genotype were verified by breeding records and RT-PCR analysis performed in the laboratory of Professor Roger Summers.
Twelve WT and 11 β3-KO male mice aged between 12 and 14 wk were used in the chronic administration study. The animals were housed individually in cages under the conditions described above. The animals were divided into three groups: WT [control (saline; n = 3); AOD (250 μg/kg·d; n = 4) and hGH (1 mg/kg·d; n = 5)] and β3-KO [control (saline; n = 3); AOD (250 μg/kg·d; n = 4); and hGH (1 mg/kg·d; n = 4)]. On d 0, all animals were anesthetized with sodium pentobarbitone and a collection of blood (200 μl) was taken in heparinized tubes (Terumo, Somerset, NJ) for glycerol determination. The plasma was isolated by centrifugation and stored at −20 C until required for analysis. For the following 28 d, the animals were given a single ip dose of compound at 0800 h each morning. Their food intake and body weight were recorded every second day and results expressed as a change from d 0. On d 28, the animals were anesthetized with sodium pentobarbitone, blood was collected for plasma glycerol determination, and they were then killed by a lethal injection of sodium pentobarbitone to the heart (35 mg/kg). Their white epididymal and brown subscapular adipose tissues were collected and weighed.
Glycerol determination
The levels of plasma glycerol were determined according to the method previously described (8) and expressed as a change from d 0 values. The amount of glycerol present in the plasma was enzymatically assayed using glycerol phosphate oxidase reactions (catalog no. GPO-337, Sigma Diagnostics, St. Louis, MO). Plasma glycerol was determined using a spectrophotometer and converted to micromoles per deciliter.
RNA extraction and RT-PCR
Tissues from ob/ob and lean mice were cut into 100-mg pieces and homogenized with 1 ml TRIzol (Life Technologies, Inc., Grand Island, NY) using a PolyTron homogenizer (Kinematica, Lausanne, Switzerland). The samples were incubated for 5 min at room temperature to permit the complete extraction of the RNA from the tissues. An aliquot of 0.2 ml chloroform was added to each milliliter of TRIzol. The method of RNA extraction was as described by the manufacturer (Life Technologies, Inc.). The final RNA pellet was air dried for 5–10 min and then resuspended in 20 μl ddH20. RNA was quantitated spectrophotometrically using the A260/280 ratio.
RT-PCR
cDNAs were synthesized by reverse transcription (RT) of 1.0 μg of each total RNA using oligo (dT)15 as a primer. The RNA in a volume of 7.7 μl H2O was heated to 70 C for 5 min then placed on ice for 2 min before the addition of a reaction mix containing 1× RT buffer (Promega Corp., Madison, WI), 1 mm deoxynucleotide triphosphates (dNTPs), 5 mm MgCl2, 18 U Rnasin (Promega Corp.), 20 U avian myeloblastosis virus RT (Promega Corp.), and 50 μg ml−1 oligo(dT)15 in a volume of 12.5 μl. Following a brief centrifugation, the reactions were incubated at 42 C for 45 min and then 95 C for 5 min. The completed RT reactions were stored at −20 C and used for PCR without further treatment.
PCR amplification was carried out on cDNA equivalent to 100 ng of starting mRNA using the following murine oligonucleotide primers (expected and observed PCR product size): β3-AR forward, 5′-TCTAGTTCCCAGCGGAGTTTTCATCG-3′; (234 bp) reverse, 5′-CGCGCACCTTCATAGCCATCAAACC-3′; β-actin forward, 5′-ATCCTGCGTCTGGACCTGGCTG-3′; (559 bp) reverse, 5′-CCTGCTTGCTGATCCACATCTGCTG-3′.
The β3-AR and actin primers were intron spanning to potentially reveal contaminant genomic DNA (none observed). Reverse primers were labeled before the PCR in a reaction mixture containing 120 pmol oligonucleotide, 70 μCi[γ -33P]ATP (Bresagen, Adelaide, Australia), 1× One-Phor-All Plus buffer (Pharmacia Biotech, Uppsala, Sweden) and 20 U T4 polynucleotide kinase (Pharmacia Biotech) in a volume of 40 μl. Following incubation at 37 C for 30 min, reactions were diluted to 100 μl with H2O and heated at 90 C for 2 min.
The PCR reaction mixture contained 1 U Taq polymerase (Life Technologies, Inc.), the supplied buffer [20 mm Tris-HCl (pH 8.4) and 50 mm KCl], 200 μm dNTPs, 2 mm Mg-acetate, 2.5 pmol of forward primer, 2.5 pmol labeled reverse primer, and cDNA in a vol of 10 μl. The PCR reactions were carried out in a Hybaid PCR Sprint machine (Hybaid, Ltd., Middlesex, UK). Following the initial heating of the samples at 95 C for 2 min, each cycle of amplification consisted of 30 sec at 95 C, 30 sec at 64 C, and 30 sec at 72 C. It was found that 24 cycles were optimum for the amplification process.
Southern blot transfer
Following amplification, PCR products were electrophoresed on 1.3% agarose gels and transferred onto Hybond N+ membranes (RPN 303B, Amersham Pharmacia Biotech) by Southern blotting in 0.4 m NaOH/1 m NaCl. The membranes were rinsed for 5 min in 0.5 m Tris-HCl (pH 7.5)/1 m NaCl and then in 0.3 m NaCl/30 mm sodium citrate, and air dried. Membranes were apposed directly to a phosphor imager screen for 18 h, and scanned using a Storm PhosphorImager and data quantitated using MCID software (Imaging Research, Inc., St. Catherines, Ontario, Canada). The β3-AR product bands were normalized against the β-actin control, averaged, and RNA isolated from treated animals was expressed against control animals.
Acute effects of AOD9604 and BRL37344 in WT andβ 3-KO mice
WT (n = 9) and β3-KO (n = 9) mice were used in this study. Animals were fasted 2 h before being individually placed in an indirect calorimeter. Calorimetry was performed as in previous studies (8). After baseline readings were taken, mice were injected with one of the following compounds: saline (control; n = 3); AOD9604 (2 mg/kg body weight; n = 3); or BRL37344 (250 μg/kg body weight; n = 3). Rates of energy expenditure, fat oxidation, and glucose oxidation were measured for an additional 30 min. The concentrations of AOD9604 and BRL37344 were determined as lowest concentration needed to give a maximal response in these mice (data not shown). Rates of energy expenditure and fat and glucose oxidation were plotted as a change from the average baseline values.
Statistical analysis
Results are expressed as the mean ± se in each experiment. The levels of significance were determined using a t test, and P values <0.05 were considered statistically significant when compared with control.
Results
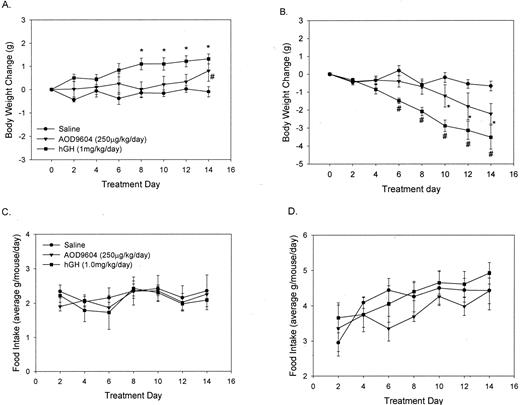
Figure 1A shows the chronic effect of saline, AOD9604, and hGH in lean C57BL/6J mice on body weight and food intake. The hGH potently increased the body weight gain of these mice, reaching significance by d 8. There was an increase in body weight after AOD9604 only on the last day of treatment. In contrast, ob/ob mice (Fig. 1B) showed a profound decrease in body weight after both AOD9604 and hGH. Importantly, these effects were not attributed to changes in food intake in either the lean or the ob/ob mice (Fig. 1, C and D, respectively).
The effect of a single daily ip dose of saline, AOD9604, or hGH on body weight changes in lean male C57BL/6J (A) or obese (ob/ob) mice (B) for 14 d. Caloric intake was recorded every second day and presented as an average for each day in lean (C) and obese (D) mice. Results are expressed as the mean ± se of six animals in each group. *, P< 0.01; #, P < 0.05, compared with saline.
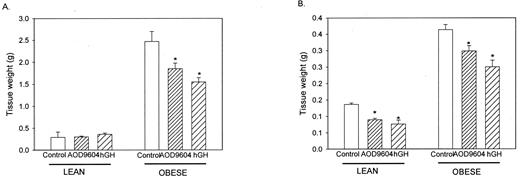
Figure 2A shows that neither hGH nor AOD9604 had any significant effect on the weight of epididymal adipose tissue in the lean mice. However, in the ob/ob mice, AOD9604 induced a 28% decrease in epididymal adipose tissue mass, and hGH induced a 40% reduction in epididymal adipose tissue mass (P < 0.05). Brown adipose tissue weights were also measured (Fig. 2B). In this tissue, both AOD9604 and hGH induced a reduction in the weight of brown adipose tissue in both the lean and ob/ob mice.
The effect of chronic 14-d administration of saline, AOD9604, or hGH on the weight of white (epididymal) adipose tissue (A) or interscapular brown adipose tissue (B) in both lean C57BL/6J and obese (ob/ob) mice. Results are expressed as the mean ± se of six tissues in each group. *, P < 0.05 vs. control.
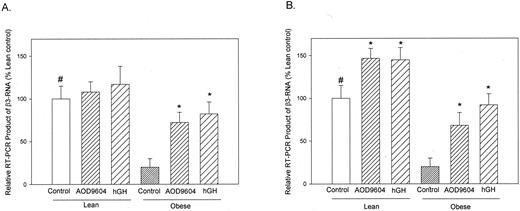
The expression of β3-AR RNA was assessed by RT by using radiolabeled primers and Southern blot analysis. Labeled bands were identified and semiquantitated by phosphorimaging. RT-PCR analysis demonstrated that ob/ob mice express lower amounts ofβ 3-AR RNA in their white (Fig. 3A) and brown (Fig. 3B) adipose tissues, compared with lean C57BL/6J mice, in agreement with others (14). Figure 3A shows the effect of saline, AOD9604, and hGH on β3-AR RNA expression in epididymal adipose tissue from both lean and obese mice, respectively. The level of β3-AR expression increased significantly in response to AOD9604 and hGH only in the obese mice, correlating with the significant decrease in epididymal adipose tissue weights seen in these mice. The same correlation was observed in brown adipose tissue, where increased expression of β3-AR was accompanied by a decrease in brown adipose tissue mass in both lean and obese mice (Fig. 3B).
A comparison of β3-AR RT-PCR expression levels in white (A) and brown (B) adipose tissues from lean and obese mice treated for 14 d with saline, AOD9604, or hGH. Results are displayed as a percentage, compared with lean controls, and expressed as the mean ± se of three determinations in each group. *, P < 0.05; #, P< 0.05 obese control vs. lean control.
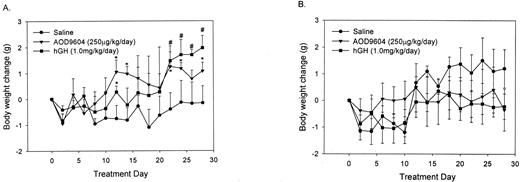
We then wanted to determine the importance of theβ 3-AR in mediating both the chronic and acute effects of AOD9604 or hGH using β3-KO mice. Theβ 3-KO mice and the control WT strain were given either AOD9604 or hGH for 28 d at the concentrations used in the previous study. As shown in Fig. 4A, both AOD9604 and hGH increased body weight after 28 d in lean WT mice similar to the effect seen after 14 d in lean C57BL/6J mice (Fig. 1A). This effect was not observed in the β3- KO mice (Fig. 4B) in whom AOD9604 or hGH had no significant effect on body weight.
The body weight change observed following 28 d intraperitoneal administration of saline (n = 3), AOD9604 (n = 4), or hGH (n = 5) in WT (A) or β3-KO mice (saline, n = 3; AOD, n = 4; hGH, n = 4) (B). Results are expressed as the mean ± se in each group. *, AOD; #, hGH; P < 0.05, compared with saline.
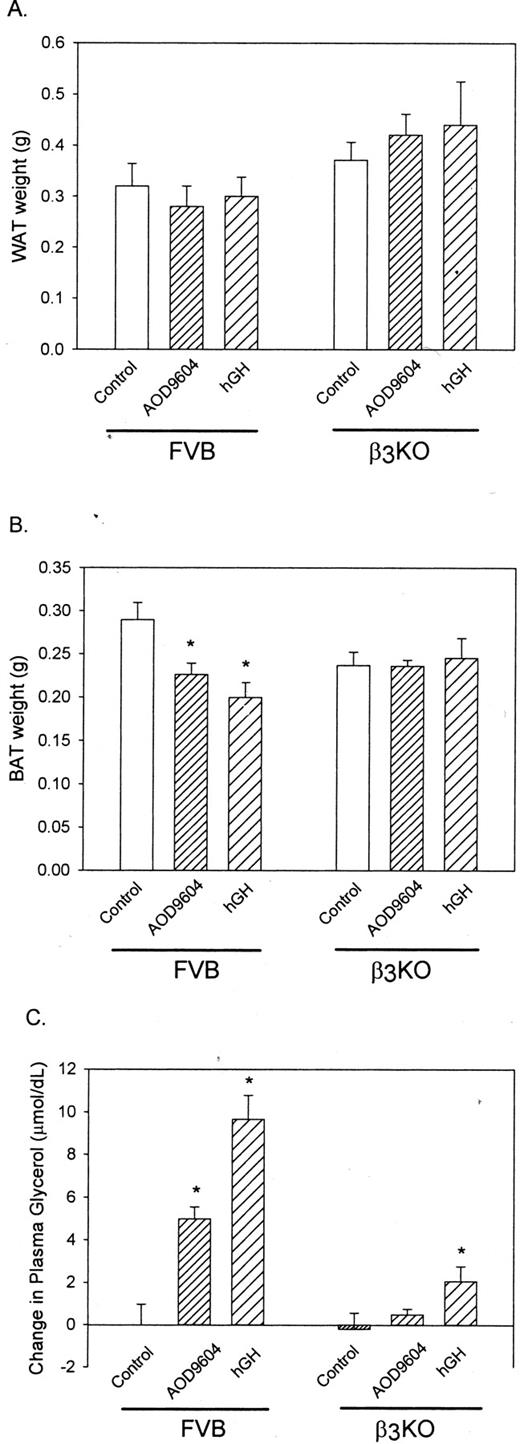
Figure 5A demonstrates that chronic administration of AOD9604 or hGH has no significant effect on the weight of white adipose tissue in either WT orβ 3-KO mice. However, in brown adipose tissue, both AOD9604 and hGH significantly reduced the size of the brown adipose tissue mass in the WT mice (Fig. 5B), by 20% and 31% (P < 0.05), respectively, as was found previously in the C57BL/6J ob/ob mice (Fig. 2B). Importantly, this effect was not observed in β3-KO mice.
The chronic effect of AOD9604 or hGH on epididymal white adipose tissue (WAT) (A) and BAT (B) weights and the change in plasma glycerol (C) in the 28-d treated WT and β3-KO mice. Results are expressed as the mean ± se of six determinations in each treatment group. *, P < 0.05 vs. control.
In this study, plasma glycerol was also assayed as a measure of lipolytic rate. As shown in Fig. 5C, both AOD9604 and hGH increased glycerol levels following treatment in the WT mice, indicative of enhanced lipolysis. In the β3-KO mouse, however, no effect was observed with AOD9604. A significant increase in plasma glycerol from controls was observed following hGH treatment, but this was markedly less than that observed in the WT mouse. These data indicate the importance of β3-AR in the lipolytic response to AOD9604 in these animals and the necessity of the receptor for the chronic effectiveness of AOD9604 and hGH on fat reduction.
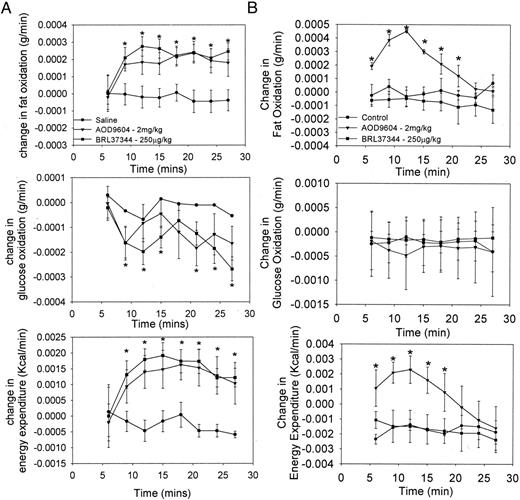
The final study involved an assessment of the acute effect of AOD9604 and a β3-AR agonist (BRL37344) on energy expenditure, fat oxidation, and glucose oxidation in WT andβ 3-KO mice. When AOD9604 or BRL37344 were administered to WT mice, an acute increase in fat oxidation and energy expenditure occurred, with an associated reduction in glucose oxidation (Fig. 6A). The effect plateaued 18 min following injection and remained stable for the duration of the experiment. The response to the two compounds was very similar, despite the fact we have previously shown that AOD9604 does not directly interact with the β3-AR as demonstrated by ligand binding studies (11). This clear separation of pathways was further confirmed in Fig. 6B in which AOD9604 clearly increases fat oxidation and energy expenditure inβ 3-KO mice, whereas BRL37344 does not. The KO mice neither decrease their glucose oxidation in response to AOD9604 nor show a prolonged increase in fat oxidation and energy expenditure in response to AOD9604.
The effect of an acute single ip injection of saline, AOD9604, or BRL37344 (β3-AR agonist) on changes in the rates of fat oxidation, glucose oxidation, and energy expenditure in WT (A) and β3-KO (B) mice. Results are expressed as the mean ± se of three determinations in each group. *, P < 0.05 vs. saline.
Discussion
It is well established that hGH is a lipolytic hormone (15), but the exact mechanisms used are still unclear. In this paper we present data that suggest that hGH and its lipolytic fragment (AOD9604) induce their chronic in vivo actions on lipolysis in part by modulating the expression of theβ 3-AR. Human GH has been shown to affect the in vivo expression and function of β-ARs in vivo in sheep (16). Data presented in this paper indicate that chronic administration of hGH influences expression of the β3-AR in adipose tissue in the ob/ob mouse. In brown adipose tissue (BAT), these compounds also increase expression of β3-AR expression in the lean C57BL/6J mouse. The increase in expression induced by chronic hGH or AOD9604 treatment correlated with the decrease in adipose tissue mass. We therefore hypothesize that treatment with either hGH or AOD9604 enhances β3-AR expression, which has been observed in murine 3T3-F442A and human SK-N-MC cells in vitro (11).
Both AOD9604 and, to a greater extent, hGH increase body weight in lean mice, compared with saline-treated animals. This is in the absence of an increase in fat mass, which suggests an increase in lean body mass occurs with these compounds. This supports previous work with hGH in rodents and humans (17). Both compounds have also been previously shown to reduce body weight and adiposity in obese mice (11). The effects of hGH and AOD9604 occur without significant changes to caloric intake. It has been reported that hGH increases, reduces, or does not change food intake in which the differences are attributed to variations in hGH preparations, concentrations, and animals used between different laboratories.
The effects of hGH and AOD9604 on fat metabolism may be mediated by an alteration in the expression of a lipolytic/antilipogenic gene. Theβ 3-AR is a major lipolytic receptor identified in rodent fat cells (18) that mediates its effects through G protein coupling to adenylate cyclase, generation of cAMP, and stimulation of PKA (19). This enzyme then phosphorylates proteins in the lipolytic cascade, including hormone-sensitive lipase (20). In BAT, the β3-AR stimulates uncoupling of the electron transport chain, enhancing the ability of mitochondria to generate heat in preference to ATP through the dissipation of the electron gradient (21). Mice that lack this receptor have lower rates of resting energy expenditure (0.0041 vs. 0.0047 kcal/min, P < 0.02) and lower rates of fat oxidation (0.00019 vs. 0.00030 g/min, P < 0.02) than control mice (data not shown).
AOD9604 and hGH appear to act in a similar manner to induce their effects on body weight regulation and adipose tissue mass in vivo. However, in vitro studies have demonstrated a number of differences suggesting that the two compounds operate via unique signaling pathways to control the regulation of theβ 3-AR. These studies suggested that AOD9604 had no interaction with the β3-AR or hGH receptors (11).
In lean animals, neither AOD9604 nor hGH had any effect on epididymal white adipose tissue mass or expression ofβ 3-AR RNA, indicating that in lean animals, this fat tissue is not a major target for these drugs in this study. In contrast, the mass of BAT in lean animals was reduced by both hGH and AOD9604, and β3-AR RNA expression was increased by both these compounds. This could possibly suggest that the increased expression of β3-ARs in brown adipocytes sensitizes catecholamines to dissipate heat.
In ob/ob mice, both AOD9604 and hGH reduced both white and brown adipose tissue mass and increased β3-AR RNA expression. This suggested that an elevation inβ 3-AR RNA expression is associated with increased fat metabolism and a reduction in the fat tissue mass in the ob/ob mouse model. Obese mice have lower levels ofβ 3-AR expression in their adipose tissues than lean mice, shown in this study and others (14). The ability of AOD9604 and hGH to increase the level ofβ 3-AR RNA expression in obese mice to a level that is comparable to those in lean mice is an exciting finding. However, it must also be considered that both hGH and AOD9604 may influence the expression of other members of the adrenergic pathway, such as the β1-ARs, hormonesensitive lipase, and signaling proteins, which are all expressed in adipose tissue and associated with lipolysis. The importance of the change inβ 3-AR expression with AOD9604 and hGH in humans is not established and will depend on the use of potent and selectiveβ 3-AR agonists that are active at the human receptor.
From this study it appears that the β3-AR is an important contributor to the effects observed on body weight in obese mice treated with AOD9604 and hGH. To determine whether theβ 3-AR is partly responsible for this effect, we examined the effects of AOD9604 and hGH in theβ 3-KO mouse. The β3-KO mouse is not grossly obese, but female mice have increased fat depots (21) and the mice do develop late-onset obesity (Summers, R. J., personal communication). AOD9604 and hGH increased body mass and decreased BAT mass in the WT strain but had no effect in the KO animals. In WT mice, plasma glycerol was increased in response to AOD9604 and hGH treatment (4 wk). However, in the KO mice, only hGH resulted in increased levels of glycerol in the KO mice, and this effect was significantly less than that observed in the WT mice. This suggests that the regulation of the β3-AR is essential in the ability of AOD9604 and hGH to mediate chronic effects on lipolysis and fat mass reduction.
The effect of AOD9604 and hGH on β3-ARs in adipose tissue is believed to be a direct action of these compounds and not an effect secondary to the fat metabolism, given that both AOD9604 and hGH can influence β3-AR expression and function in a nonadipocyte human cell line (11). Hence, the β3-AR appears to be necessary for the chronic effectiveness of AOD9604 on lipolysis in BAT.
The acute effect of AOD9604 and BRL37344 (aβ 3-AR agonist) on energy expenditure and substrate oxidation rates in WT and KO mice was also assessed. KO animals had lower energy expenditure, lower fat oxidation, and increased glucose oxidation, compared with the WT controls (data not shown). Injection of WT mice with a single dose of BRL37344 or AOD9604 increased energy expenditure and fat oxidation and decreased glucose oxidation. In the KO animals, BRL37344 failed to elicit any response in these metabolic parameters, clearly demonstrating that its effects are mediated exclusively through the β3-AR. AOD9604 did elicit a response in the KO mice, increasing fat oxidation and energy expenditure, although the response was not as great as in WT mice, suggesting that β3-ARs are not responsible for the acute biological response of AOD9604 on lipid metabolism. This is consistent with our previous findings in which AOD9604 was shown not to bind to the β3-AR (11). The size and duration of the metabolic responses to AOD9604 in the β3-AR KO animals was different from that observed in the control wild-type mice. The response was more rapid, shorter in duration, and greater in peak response. This may be because the KO animals are more acutely sensitive to lipolytic agents, a compensation for the ablation of the major lipolytic receptor.
These findings suggest that the acute effects of AOD9604 are quite different from the chronic effects. Enhancedβ 3-AR expression appears to play a major role in the chronic effectiveness of the compound in terms of fat metabolism and weight loss. The acute effects observed in this study confirm that the β3-AR is not the sole mediator of this action. The increase in β3-AR expression in response to hGH and AOD9604 would permit enhanced lipolytic sensitivity. Identification of the components of the intracellular pathway(s) and effector(s) activated by AOD9604 are currently being investigated. The results presented in this paper suggest that the effectiveness of AOD9604 and hGH may partly rely on their ability to increase levels of β3-AR RNA expression in models of obesity in which the numbers of the lipolytic receptor are low. These unique properties may give AOD9604 an advantage over other lipolytic agonists such as adrenergic agents and hGH, which exhibit undesirable side effects when administered chronically (22).
Acknowledgements
This work was supported by Metabolic Pharmaceuticals (Pty Ltd.), Melbourne, Australia.
Abbreviations
- β3-AR
β3-Adrenergic receptor
- β3-KO
β3-AR knock-out
- BAT
brown adipose tissue
- hGH
human GH
- ob/ob
obese
- RT
reverse transcription/transcriptase
- WT
wild-type.