-
PDF
- Split View
-
Views
-
Cite
Cite
Bryan Korithoski, Céline M. Lévesque, Dennis G. Cvitkovitch, The involvement of the pyruvate dehydrogenase E1α subunit, in Streptococcus mutans acid tolerance, FEMS Microbiology Letters, Volume 289, Issue 1, December 2008, Pages 13–19, https://doi.org/10.1111/j.1574-6968.2008.01351.x
Close - Share Icon Share
Abstract
Streptococcus mutans, an etiological agent of dental caries, is a normal inhabitant of dental plaque. Two main virulence factors of S. mutans are acidogenicity and aciduricity – the ability to produce acid and survive at low pH, respectively. Metabolic processes, including the catabolism of pyruvate, are finely regulated following acid exposure in S. mutans. Proteome analysis of the S. mutans acid response has shown pyruvate dehydrogenase A (PdhA) is upregulated. PdhA is the E1αsubunit of the four-enzyme pyruvate dehydrogenase complex, responsible for the heterofermentative catalysis of pyruvate into acetyl-CoA. Acetyl-CoA is subsequently catalyzed into ethanol and acetate yielding additional ATP. This investigation examined the relationships between PdhA, aciduricity, and metabolism in S. mutans. An S. mutans pdhA knockout (PDHAKO) revealed an acid sensitive phenotype, displayed by increased doubling times, and decreased competitiveness in a biofermentor. Quantitative real-time PCR showed pdhA expression increased dramatically during acidic growth and under acid adaptation. Additionally, pdhA expression responded to conditions favoring heterofermentative growth; decreased in the presence of excess glucose, and increased during stationary phase compared with mid-log phase growth. This study demonstrated that in S. mutans, pdhA expression responds to conditions conducive to heterofermentation and deletion of pdhA resulted in decreased aciduricity.
Introduction
According to the World Health Organization, dental caries is still a major oral health problem in industrialized countries affecting up to 90% of children (Petersen, 2003). Dental caries is the dissolution of tooth enamel caused by the acidic end products resulting from bacterial metabolism of dietary carbohydrates. Streptococcus mutans, a ubiquitous constituent of human dental plaque, is the main bacterial species associated with the initiation and progression of dental caries. Streptococcus mutans has the ability to rapidly metabolize a variety of carbohydrates into lactate – a highly acidic organic acid end product of glycolysis. Furthermore, S. mutans has developed a complex response network allowing it the ability to survive in the acidic environment created by its own acid production. These two previously mentioned characteristics, termed acidogenicity and aciduricity, respectively, are vital cariogenic determinants of this bacterial species (reviewed by Banas, 2004). During periods of high carbohydrate concentration and low pH, acidogenicity and aciduricity allow S. mutans to dominate over other organisms within the dental plaque biofilm, leading to the development and progression of carious lesions.
When S. mutans is grown in excess glucose, lactate is the major fermentation product. However, under conditions of glucose limitation, ethanol, acetate, and formate, in conjunction with lactate are the resulting end products of metabolism (Carlsson & Hamilton, 1994). This divergence in metabolism occurs at pyruvate, which has been shown to be a key metabolic control node in many bacterial species (Guest et al., 1989; Cocaign-Bousquet et al., 1996; Modak et al., 2002). Pyruvate can be converted to lactate via lactate dehydrogenase (homofermentation) or to formate, ethanol, and acetate via pyruvate–formate lyase (heterofermentation) (Carlsson & Hamilton, 1994). A third alternative pathway of pyruvate catabolism is via the pyruvate dehydrogenase complex (PDH) in which pyruvate is oxidized into acetyl-CoA and CO2 (Carlsson et al., 1985). Acetyl-CoA is further catabolized into ethanol, via alcohol dehydrogenase, or acetate, via acetate kinase.
In gram-positive bacteria, the PDH is composed of four distinct polypeptides E1α, E1β, E2, and E3 (Snoep et al., 1992). These proteins combine to from an icosahedral complex consisting of pyruvate dehydrogenase (E1 subunits), dihydrolipoyl transacetylase (E2), and dihydroipoamide dehydrogenase (E3) (Modak et al., 2002).
The activity of PDH in gram-negative bacteria depends largely on allosteric regulations; however, in gram-positive bacteria, PDH seems to be regulated by redox potential (Snoep et al., 1992). Such is the case in Lactococcus lactis, due to pyruvate formate lyase (PFL) being extremely oxygen sensitive, PDH is responsible for pyruvate metabolism in an aerobic environment (Snoep et al., 1992). In Enterococcus faecalis, however, both PFL and PDH are active under anaerobic conditions, analogous to pyruvate oxidation in the gram-negative bacterium Klebsiella pneumoniae (Snoep et al., 1993; Menzel et al., 1997). A previous report in S. mutans described increased PDH activity in response to an aerobic environment (Carlsson et al., 1985).
Human dental plaque routinely goes through cycles of carbohydrate excess and limitation, and therefore metabolic regulation between homofermentation and heterofermentation is paramount to the survival of S. mutans. Heterofermentation is thought to play an important role in the acid tolerance of S. mutans as the metabolic endproducts are weaker organic acids and heterofermentation yields an additional ATP molecule per pyruvate molecule (Carlsson & Hamilton, 1994). During acid challenge, in S. mutans, the protein expression levels of the E1αsubunit (termed PdhA) were shown to increase (Welin et al., 2003). These data suggest that PDH may play an important role in the acid tolerance of S. mutans. The present study looks to address the involvement of PDH, specifically pdhA, in the metabolism and acid tolerance of S. mutans.
Materials and methods
Bacterial strains and growth conditions
The S. mutans UA159 strain was used in this study. The pdhA isogenic mutant (PDHAKO) was generated using PCR ligation mutagenesis (Lau et al., 2002). Primers used for mutagenesis are shown in Table 1. Streptococcus mutans UA159 cells were grown in Todd Hewitt broth supplemented with 0.3% yeast extract (THYE). Erythromycin was added to a final concentration of 10 μg mL−1 as needed.
Primers used in this study
Restriction sites are underlined: AscI GGCGCGCC; FseI GGCCGGCC.
Primers used in this study
Restriction sites are underlined: AscI GGCGCGCC; FseI GGCCGGCC.
Growth kinetics
Overnight cultures of UA159 and PDHAKO were diluted (1 : 20) into fresh THYE and incubated at 37 °C under an atmosphere of 5% CO2 until cultures reached mid-logarithmic growth phase (OD600 nm∼0.4). These subcultures were subsequently inoculated 1 : 20 into microtitre plates in quadruplicate containing THYE broth at pH 7.5 and pH 5.0. Cultures were incubated at 37 °C for 24 h and growth followed using an automated growth reader (Bioscreen C, Labsystems, Finland). Growth curves were generated and doubling times (Td) calculated from the data.
Acid tolerance response (ATR) assay
Overnight cultures of S. mutans UA159 and PDHAKO strains were diluted (1 : 20) into TYE (10% tryptone, 5% yeast extract, 17.2 mM K2HPO4) with additional 5 mM glucose at pH 7.5 and incubated at 37 °C, in a 5% CO2 enhanced environment until mid-log growth phase was achieved (OD600 nm∼0.4). Cultures were divided into two aliquots, termed ‘adapted’ and ‘nonadapted,’ and pelleted by centrifugation. Nonadapted cells were immediately resuspended in TYE at the killing pH of 3.2. An aliquot was immediately removed (t=0) and serially diluted in 10 mM potassium phosphate buffer (pH 7.2) and incubation continued for 3 h at 37 °C in an atmosphere of 5% CO2. Each dilution was spotted in triplicate (20 μL per spot) onto THYE agar plates and incubated at 37 °C, in a 5% CO2 supplemented atmosphere for 2 days. Adapted cells were resuspended in TYE at pH 5.5 for 2 h before being subjected to the killing pH (3.2). The ATR was expressed as the percentage of cells to survive the lethal pH at 1 h, 2 h, and 3 h compared with the number of cells present at time 0.
Terminal pH
Overnight cultures of S. mutans UA159 and PDHKO strains (eight independent cultures of each strain) were diluted (1 : 40) in THYE at pH 7.5, pH 6.0, and pH 5.0 and incubated at 37 °C in an atmosphere of 5% CO2 for 20 h before recording the terminal pH.
Measurement of glycolytic rates
Overnight cultures were diluted (1 : 10) into THYE at pH 7.5 and incubated at 37 °C until mid-log growth phase was reached (OD600 nm∼0.4). The cells were subsequently pelleted via centrifugation and washed twice with ice-cold PK solution (1% peptone, 1% KCl) at either pH 7.0 or pH 5.0. Cells were then resuspended in PK solution at the appropriate pH to yield an OD600 nm∼1.0. Aliquots (18 mL) of cell suspension were equilibrated in the reaction vessel at 37 °C until residual glycolytic activity had diminished. After equilibration, the reaction was initiated with the addition of 200 mM glucose. Glycolytic rates were monitored by the rate of addition of potassium hydroxide (10 mM KOH at pH 7.0 and 2 mM KOH at pH 5.0) required to keep the pH constant utilizing a Radiometer ABU901 autoburrette in conjunction with a PHM290 pH controller (Radiometer, Denmark). The glycolytic rates are defined as micromoles of acid neutralized per milligram (dry weight) of cells per minute.
Continuous culture acidic competition assay
Equal numbers of UA159 and LGLKO were simultaneously inoculated (each at a 1 : 80 ratio) into a chemostat biofermentor previously charged with 1/4 THYE+5 mM glucose at pH 6.0 and containing glass rods for biofilm accumulation. Cells were continuously cultured as described previously with some modification (Li & Bowden, 1994). Bacterial cultures were initially established at pH 6.0 and a fresh media flow rate of 0.1 dilutions h−1 (D) for 18 h. After culture establishment, culture pH was reduced to pH 5.0 and flow rate increased to D=0.5. Aliquots of planktonic cells were removed, serially diluted and plated onto THYE agar and THYE+10 μg mL−1 erythromycin agar. Biofilm cells were quantified by the removal of glass rods. Biofilm cells were released by vortex and cells serially diluted and plated.
Cell preparation for gene expression analysis
Streptococcus mutans UA159 cells were grown under the following conditions before RNA isolation. Acid growth: Overnight cultures of S. mutans cells were diluted (1 : 40) in THYE (pH 7.5 and pH 5.0), and incubated at 37 °C in an atmosphere of 5% CO2 until mid-log growth phase was reached (OD600 nm∼0.4). Acid adaptation: Overnight cultures of S. mutans UA159 cells were diluted (1 : 20) in TYE pH 7.5 and incubated until mid-log growth phase in a 37 °C, 5% CO2 environment. Cells were divided into two aliquots, centrifuged, and subsequently resuspended to their original concentration in TYE pH 5.0. RNA from one aliquot was processed immediately, and the second aliquot was incubated at 37 °C supplemented with 5% CO2 for 2 h before RNA extraction. Oxygen environment: Overnight cultures were diluted (1 : 20) into TYE pH 7.5 supplemented with 5 mM glucose that had been pre-equilibrated in an anaerobic chamber (10% CO2, 10% H2, and balanced N2) as well as into TYE pH 7.5 with supplemented 5 mM glucose preincubated at 37 °C under standard atmospheric conditions. RNA was harvested from the cultures after cells reached mid-log phase (OD600 nm∼0.4). Biofilm growth: Overnight cultures were inoculated 1 : 100 into 24-well polypropylene microtitre plates containing 1/2 × concentrated THYE pH 5.0 supplemented with 5 mM glucose. Cells were allowed to grow at 37 °C with 5% CO2 for 18 h. After incubation, planktonic cells were separated from biofilm cells and RNA was extracted from each. Growth phase: Overnight cultures of S. mutans UA159 cells were diluted (1 : 20) into TYE at pH 7.5 and incubated at 37 °C with 5% CO2 supplementation. RNA was harvested from the cultures when the cells reached mid-log growth phase (OD600 nm∼0.4) and after reaching stationary phase (20 h). Glucose response: Cell suspensions of UA159 were prepared at both pH 7.0 and pH 5.0 as described for measurement of glycolytic rates. Cells were equilibrated for 20 min at 37 °C to eliminate residual glycolytic activity. Subsequent to equilibration, 200 mM glucose was added to the suspensions for 15 min before RNA isolation.
Quantitative real-time PCR (qRT-PCR) analysis of pdhA RNA expression
Total RNA was harvested and treated with RQ1 RNAse-free DNAse (Promega) as described previously (Hanna et al., 2001). From this RNA, cDNA was generated via reverse transcription using a First Strand cDNA synthesis kit (MBI Fermentas) according to the manufacturer's instructions. RNA samples lacking reverse transcriptase were incorporated as controls to assure that the results were not the product of residual DNA contamination. These single stranded cDNA templates were used for qRT-PCR reactions that were carried out using the QuantiTect SYBRGreen PCR kit (Qiagen) in a Mx3005P QPCR system (Stratagene). Specific primer sequences used (Table 1) for the reactions were designed to yield 100–150 bp products. For each reaction, the cycle threshold (CT) was measured; this value was inversely proportional to the starting amount of target DNA in each sample. All data was normalized against the expression of an internal standard. For all experiments 16S rRNA was used, except gyrA was the internal standard used for biofilm experiments, as the expression of these genes was shown to be stable under experimental conditions. The expression fold change was determined using the 2−ΔΔCT method (Livak & Schittgen, 2001).
Results
The involvement of PdhA in aciduricity
The phenotypic effect of the pdhA knockout on S. mutans was first determined by measurements of growth rates at both pH 7.5 and pH 5.0. The PDHAKO mutant and the UA159 parent displayed similar growth kinetic when grown at pH 7.5 (mean Td's of 68.2±1.1 and 70.4±0.2 min, respectively). Under both pH growth conditions, the final growth yield was the same for both UA159 and the mutant PDHAKO. However, the PDHAKO strain displayed significantly slower growth than parent UA159 when grown at pH 5.0 (mean Td's of 190.4±6.3 and 164.3±5.2 min, respectively).
The involvement of pdhA in the aciduricity of S. mutans was further investigated by employing ATR assays for comparing the PDHAKO and UA159 strains (Fig. 1). The PDHAKO strain displayed reduced innate survival capacity compared with UA159 under nonadapted conditions (0.00001% and 0.0015% survival for PDHAKO and UA159, respectively). PDHAKO cells that were acid adapted, by exposure to pH 5.5 media before killing pH 3.2 media, also had significantly reduced survival ability compared with UA159 (0.00038% and 0.131% survival for PDHAKO and UA159, respectively).
Acid tolerance response of Streptococcus mutans UA159 and PDHAKO strains. Cells were grown in TYE supplemented with glucose at pH 7.5 to mid-log phase and incubated in TYE pH 5.5 for 2 h and then subjected to TYE pH 3.2 [adapted UA159 (▪) and PDHAKO (□)] or immediately subjected to TYE pH 3.2 [nonadapted UA159 ( ), PDHAKO (
), PDHAKO ( ]. Percentage of cell survival was calculated as the CFU mL−1 at a given time divided by the CFU mL−1 at time zero. *Statistical significance comparing nonadapted cells (P<0.05); †statistical significance comparing adapted cells (P<0.05).
]. Percentage of cell survival was calculated as the CFU mL−1 at a given time divided by the CFU mL−1 at time zero. *Statistical significance comparing nonadapted cells (P<0.05); †statistical significance comparing adapted cells (P<0.05).
Terminal culture pH was measured for UA159 and PDHAKO. PDHAKO was unable to acidify cultures to the same degree when compared with UA159. This phenomenon became more pronounced as the initial culture pH was reduced. With initial pH of the medium reduced to pH 5.0, the mean terminal pH for UA159 and PDHAKO were 4.118±0.0027 and 4.149±0.0025 (statistical significance determined using single factor anova, P=1.47 × 10−6).
Simultaneous biofermentor competition
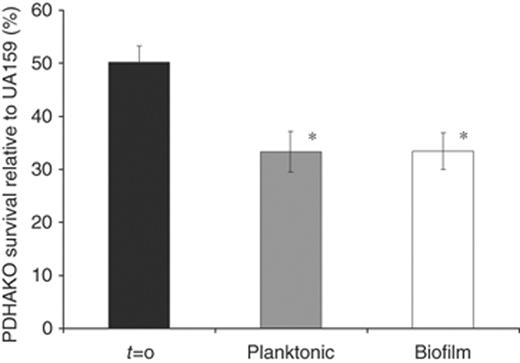
Streptococcus mutans UA159 and PDHAKO were grown in simultaneous competition in an acidic environment in a continuous culture biofermentor (Fig. 2). After only 48 h of growth competition, a significant reduction in PDHAKO cells relative to parent strain UA159 present in both planktonic and biofilm fractions was observed.
Biofermentor growth competition between UA159 and PDHAKO. Cultures were initiated at pH 6.0 before exposure to pH 5.0. Percentage of PDHAO survival in both biofilm (□) and planktonic ( ) culture fractions was calculated as the CFU mL−1 present on THYE+10 μg mL−1 erythromycin agar plates divided by the CFU mL−1 present on THYE agar. The results are expressed as the mean±SE of three independent experiments. *Statistical significance compared with t=0 (P<0.05).
) culture fractions was calculated as the CFU mL−1 present on THYE+10 μg mL−1 erythromycin agar plates divided by the CFU mL−1 present on THYE agar. The results are expressed as the mean±SE of three independent experiments. *Statistical significance compared with t=0 (P<0.05).
The effect of PdhA on glycolytic rates
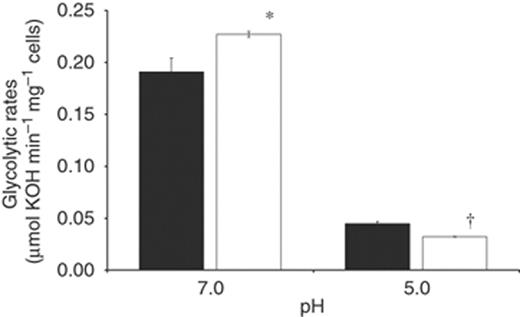
The effect of pdhA inactivation, thought to play a vital role in pyruvate metabolism, on glycolytic rates of S. mutans was investigated at both pH 7.0 and pH 5.0 (Fig. 3). At pH 7.0, the absence of pdhA resulted in an increased glycolytic rate for PDHAKO over that of UA159 (0.23 and 0.19 μmol min−1 mg−1, respectively). An inverse trend was observed when the reaction pH was decreased to pH 5.0, when the deletion of pdhA resulted in a decreased glycolytic rate for PDHAKO (32.2 nmol min−1 mg−1) compared with UA159 (44.9 nmol min−1 mg−1).
Glycolytic rates of Streptococcus mutans UA159 and PDHAKO. Glycolytic rates for UA159 (▪) and PDHAKO (□) were monitored by measuring the addition rate of 10 mM KOH to cell suspensions following the addition of 200 mM glucose at pH 7.0 and pH 5.0. Results are expressed as the mean±SE of three independent experiments. *Statistical significance relative to UA159 pH 7.0 (P<0.05); †statistical significance relative to UA159 pH 5.0 (P<0.05).
The expression profile of pdhA
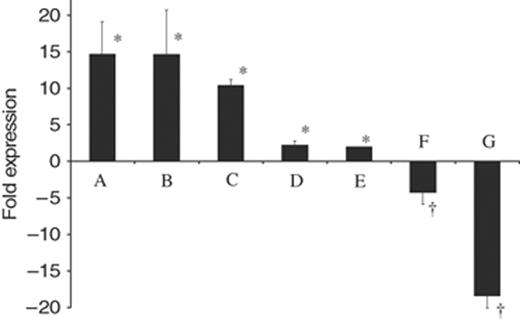
The differences in expression of S. mutans pdhA, at the mRNA level, were quantified using qRT-PCR under various environmental conditions relating to aciduricity, growth phase, and exogenous glucose. Results from these experiments are presented in Fig. 4. In liquid culture, pdhA levels escalated 14.7-fold in cells grown at pH 5.0 compared with those grown at pH 7.5. A similar trend was observed when cells were exposed to pH 5.5 for 2 h; pdhA RNA increased 14.6-fold during this period. Transcription of pdhA was dramatically higher in stationary growth phase cells compared with logarithmic growth phase cells, increasing 10.4-fold. The transcriptional response of S. mutans pdhA levels in reaction to excess glucose was assessed via qRT-PCR. Transcription of pdhA mRNA decreased significantly in the presence of excess glucose at both pH 7.5 and pH 5.0, diminishing by 18.4-fold and 4.3-fold, respectively. Additionally, pdhA had a twofold increase in expression in acidic biofilm cells over planktonic cells.
Differential gene expression of pdhA during various environmental stresses. Expression fold was standardized to 16S rRNA gene expression. Conditions investigated: column A, growth at pH 5.0 vs. pH 7.5; column B, 2 h acid adaptation at pH 5.5; column C, stationary vs. mid-log growth phase; column D, anaerobic vs. aerobic growth; column E, biofilm vs. planktonic growth at pH 5.0; column F, response to the presence of 200 mM glucose at pH 5.0; column G, response to the presence of 200 mM glucose at pH 7.0. Results are expressed as the mean±SE of at least four independent experiments. *Statistical significance, expression fold >1 (P<0.01); †statistical significance, expression fold<−1 (P<0.01)
Discussion
Streptococcus mutans is a frequent inhabitant in human dental plaque, and is the species most commonly associated with dental caries. Carious lesions are the end result of dissolution of tooth enamel by the carboxylic acid end products of dietary carbohydrate metabolism by oral bacteria, especially S. mutans. In order to survive this dramatic pH drop, S. mutans has developed a complex network of stringently regulated, coordinated responses affording it the abilities of varied carbohydrate metabolism and acid survival (Banas, 2004). The focus of this investigation was to discern the role of pyruvate dehydrogenase, specifically PdhA, in the acid tolerance of S. mutans and its expression relating to heterofermentation.
In S. mutans, the switch to mixed acid metabolism results in increased concentrations of ethanol and acetate as end products (Carlsson & Hamilton, 1994). We propose PDH to be pivotal to the regulation of acid production in S. mutans; therefore, investigations into the effects of a pdhA knockout on glycolysis were undertaken by measuring glycolytic rates and by utilizing qRT-PCR. Our results revealed drastic decreases in pdhA transcription in the presence of excess glucose at both pH 7.0 and pH 5.0, indicating that pdhA was almost entirely repressed during conditions conducive to homofermentation. Additionally, pdhA expression was monitored in response to growth phase, displaying dramatically increased expression in stationary growth phase over logarithmic phase, further supporting pdhA involvement in homofermentative pyruvate metabolism in S. mutans. In the absence of pdhA, the rates of glycolysis increased at pH 7.0 and decreased at pH 5.0 in PDHAKO relative to UA159. These results indicate that PdhA aids in S. mutans metabolic regulation over a broad pH range.
The PDH complex is involved in the switch from homofermentation to heterofermentation in many bacterial species. PFL in S. mutans has been shown to be extremely sensitive to oxygen (Takahashi et al., 1987; Takahashi-Abbe et al., 2003). It has been shown in other lactic acid bacteria that the PDH complex facilitates heterofermentation that is accomplished in an oxygen-rich environment. As such, we hypothesized that the PDH complex would be important for S. mutans heterofermentation, and this importance would be paramount in the presence of oxygen (Snoep et al., 1992). This hypothesis was tested by monitoring the level of pdhA transcription under various environmental conditions via qRT-PCR. Interestingly, expression of pdhA was elevated in an anaerobic environment compared with an aerobic environment, contrary to results seen in L. lactis. These data suggested that PDH and pyruvate formate lyase are both responsible for pyruvate heterofermentation under anaerobic conditions, while PDH is solely responsible in an aerobic environment.
Previous investigations into the proteome-wide response of S. mutans to acid challenge have revealed a vast and diverse list of proteins with altered expression profiles (Wilkins et al., 2002; Welin et al., 2003; Len et al., 2004a, b). Work conducted by Welin et al. (2003) investigating the translation response of S. mutans to acid challenge via 2D gel electrophoresis revealed a number of proteins with altered expression profiles. This work showed PdhA to be upregulated by acid challenge 3.5- and 2.5-fold in planktonic and biofilm cells, respectively. To investigate mRNA expression of pdhA under acidic conditions we implemented qRT-PCR for this study. Our data clearly showed dramatic increases in pdhA mRNA levels during both acidic growth and acid adaptation.
The involvement of PdhA in the acid tolerance of S. mutans is further supported by our growth kinetics findings that the PDHAKO mutant strain had statistically significant slower doubling times at pH 5.0 compared with UA159. The PDHAKO mutant was also unable to acidify culture media to same extent as UA159. Additionally, the PDHAKO strain was far more acid sensitive compared with UA159 under the conditions of the ATR assay. These data illustrate the acid-sensitive phenotype resulting from pdhA deletion in S. mutans. These findings, together with the qRT-PCR results under acid challenge suggest PdhA is an important component of aciduricity in S. mutans.
This study examined the involvement of the PdhA in heterofermentation, and the aciduricity of S. mutans. Our data clearly indicated expression of pdhA was dramatically increased under conditions conducive to mixed acid metabolism and decreased during conditions favoring lactic acid metabolism. Additionally, the deletion of pdhA resulted in an acid-sensitive phenotype suggesting a link between PdhA and the acid tolerance afforded to S. mutans. The ability to inhibit or downregulate pdhA could be a novel strategy to reduce the acid tolerance of S. mutans and hence reduce its associated cariogenicity. This work adds to our further understanding of S. mutans' robust metabolic flexibility that has evolved to facilitate its domination in the low pH environment of carious dental plaque.
Acknowledgements
The authors would like to thank Richard Mair for help with PCR primer design. This work was supported by an operating grant from the Canadian Institutes for Health Research MT-15431. D.G.C. is the recipient of a Canada Research Chair. B.K. is a CIHR strategic training fellow in Cell Signaling in Mucosal Inflammation & Pain (STP-53877, CIHR MT-1).
References
Editor: William Wade



![Acid tolerance response of Streptococcus mutans UA159 and PDHAKO strains. Cells were grown in TYE supplemented with glucose at pH 7.5 to mid-log phase and incubated in TYE pH 5.5 for 2 h and then subjected to TYE pH 3.2 [adapted UA159 (▪) and PDHAKO (□)] or immediately subjected to TYE pH 3.2 [nonadapted UA159 (), PDHAKO (]. Percentage of cell survival was calculated as the CFU mL−1 at a given time divided by the CFU mL−1 at time zero. *Statistical significance comparing nonadapted cells (P<0.05); †statistical significance comparing adapted cells (P<0.05).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsle/289/1/10.1111_j.1574-6968.2008.01351.x/2/m_FML_1351_f1.jpeg?Expires=1716339371&Signature=CCRx18~Mmln3~jcv8ohN8jWgQHYF-dYOMr94SZjsiJ2gKIuH63djxbMPXaTCa8MJCsysszXGycnPs1QtbhUJljmoYs-3rNlBMZRrTzNouyRvYgskf63jg4GocPnr4WsOP~h3Cs~TFDyAXi7qkLU0noJ5Tsf67oZ18xtUrN8UBssvO1neIxOLfTehtcLE53SWDqUs97L~pKDcdUhRvAqLppmh~45691CgONpn51O2CtWcBkqqJJiibaxCC9CKsowKF1f4ecLJIC~OzPODvCkRygKyVNm14CIvW3F2~QAznSy7ZJ26PCtVogSGrl5c~M0JpFkEeEP3eEyCbdw6w6dazQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)