-
PDF
- Split View
-
Views
-
Cite
Cite
Chia-Yu Chen, Chieh-Hsiang Chan, Chun-Ming Chen, Yin-Shuan Tsai, Tsung-Yuan Tsai, Yan-Hwa Wu Lee, Li-Ru You, Targeted inactivation of murine Ddx3x: essential roles of Ddx3x in placentation and embryogenesis, Human Molecular Genetics, Volume 25, Issue 14, 15 July 2016, Pages 2905–2922, https://doi.org/10.1093/hmg/ddw143
Close - Share Icon Share
The X-linked DEAD-box RNA helicase DDX3 (DDX3X) is a multifunctional protein that has been implicated in gene regulation, cell cycle control, apoptosis, and tumorigenesis. However, the precise physiological function of Ddx3x during development remains unknown. Here, we show that loss of Ddx3x results in an early post-implantation lethality in male mice. The size of the epiblast marked by Oct3/4 is dramatically reduced in embryonic day 6.5 (E6.5) Ddx3x−/Y embryos. Preferential paternal X chromosome inactivation (XCI) in extraembryonic tissues of Ddx3x heterozygous (Ddx3x−/+) female mice with a maternally inherited null allele leads to placental abnormalities and embryonic lethality during development. In the embryonic tissues, Ddx3x exhibits developmental- and tissue-specific differences in escape from XCI. Targeted Ddx3x ablation in the epiblast leads to widespread apoptosis and abnormal growth, which causes embryonic lethality in the Sox2-cre/+;Ddx3xflox/Y mutant around E11.5. The observation of significant increases in γH2AX and p-p53Ser15 indicates DNA damage, which suggests that loss of Ddx3x leads to higher levels of genome damage. Significant upregulation of p21WAF1/Cip1 and p15Ink4b results in cell cycle arrest and apoptosis in Ddx3x-deficient cells. These results have uncovered that mouse Ddx3x is essential for both embryo and extraembryonic development.
Introduction
RNA helicases of the DEAD-box family are found in all species, from bacteria to human. Comparative sequence and structural analyses together with biochemical and biophysical studies reveal that DEAD-box proteins contain a helicase core that harbors highly conserved ATP-binding and RNA-binding sites (1). DEAD-box proteins act in conjunction with specific RNA substrates or interacting partners, and affect essentially all cellular functions including transcription, recombination, DNA repair, pre-mRNA splicing, RNA turnover, nuclear export, ribosome biogenesis, translation and posttranslational regulation (2,3). Studies on DEAD-box proteins of model organisms report that they have multiple functions. For example, targeted inactivation of Ddx20 in mice results in early embryo lethality before the four-cell stage, clearly indicating the physiological significance of the DEAD-box RNA helicase during early embryo development (4). Although the DEAD-box RNA helicase subunits p68 (Ddx5) and p72 (Ddx17) are ubiquitously detected in developing mouse embryos, knockout of p68 and p72 causes lethality around embryonic day 11.5 (E11.5) and postnatal day 2 (P2), respectively (5). Several hundred DEAD-box RNA helicases have been identified. Loss-of-function studies suggest that DEAD-box RNA helicase members are functionally non-redundant and involved in distinct biological responses. Only a few DEAD-box RNA helicase genes have been characterized in vivo.
DDX3X (also known as DDX3, DBX, and CAP-Rf) is a member of the DEAD-box RNA helicase family and is located on human X chromosome p11.3 to p11.23 (6,7). The DDX3X homolog DDX3Y is located in the non-recombining region of the human Y chromosome. DDX3X expression escapes X-inactivation in females (8). Several studies report that DDX3X participates in a variety of gene regulation events, including transcriptional regulation, RNA unwinding, splicing, RNA nuclear export, ribosomal biogenesis, and mRNA translation. These wide-ranging activities have been associated with a broad spectrum of biological processes, such as cell cycle/cell growth, apoptosis, tumorigenesis, innate immunity and virus replication (9–12). However, the results from studies using different cell lines and cancer specimens to elucidate DDX3X function have not been consistent and have generated some debate. Recent studies have identified DDX3X mutations in the individuals with developmental disorders (13) and intellectual disability (ID)/developmental delay (14). The differences in disease transmission and phenotypic variations among affected females and males suggest that DDX3X exerts dose-dependent and gender-specific effects in normal development and diseases. Therefore, more evidence is required to validate the in vivo function of DDX3X.
Here, we perform an in vivo analysis of Ddx3x in the mouse. We show that Ddx3x function is necessary for embryonic development and placentation. Loss of Ddx3x leads to early post-implantation lethality of the Ddx3x−/Y mutant. Paternal X-inactivation of Ddx3x affects trophoblast development and vascularization in extraembryonic tissues and embryo survival of Ddx3x−/+ females with a maternally inherited Ddx3x null allele. Epiblast-specific knockout of Ddx3x leads to DNA damage and profound cell cycle arrest, which prohibits early embryo development.
Results
Targeted disruption of Ddx3x causes early embryo lethality in male mice and perinatal lethality in a Ddx3x−/+ mutant with a maternal null allele
To generate a floxed Ddx3x allele (Supplementary Material, Figure S1), two loxP sites were placed in intron 3 and exon 17 of Ddx3x genomic DNA using recombineering technology (15). A Prm-cre transgenic mouse line (16) was used to excise the floxed Ddx3x sequence in the germline of Prm-cre/+;Ddx3xflox/Y male mice. These males were bred with wild-type (Ddx3x+/+) females to generate the first generation (F1) of Ddx3x heterozygous (Ddx3x+/−) mice. In this cross scheme, we obtain only female mice with a null allele because Ddx3x is located on the X chromosome.
The F1 Ddx3x+/− females were born at the expected Mendelian ratio, developed normally, and were indistinguishable from their littermate controls. Subsequently, F1 Ddx3x+/− females were crossed with wild-type males (Ddx3x+/Y) to produce the second generation (F2) of Ddx3x mutant mice. Considering the parental origin of Ddx3x allele, the heterozygous Ddx3x female mutants with maternally and paternally derived null alleles are described as Ddx3x−/+ and Ddx3x+/−, respectively. Offspring numbers and genotypes are summarized in Table S1 (Supplementary Material, Table S1). The maternally derived Ddx3x mutant allele could not be passed to the F2 generation and resulted in 100% lethality for both male and female mice (Supplementary Material, Table S1). Only one progressively necrotic Ddx3x−/Y null male (1 of 80 embryos from 10 litters) was obtained at E9.5 (Supplementary Material, Table S1 and Figure S2). At E13.5 and E14.5, Ddx3x−/+ females were readily obtained. At E16.5−E18.5, the proportions of Ddx3x−/+ females were dramatically reduced to 5/64 (7.8%). These results indicate that loss of the maternally derived Ddx3x allele causes early post-implantation lethality in male mice and prenatal lethality in Ddx3x−/+ female mice.
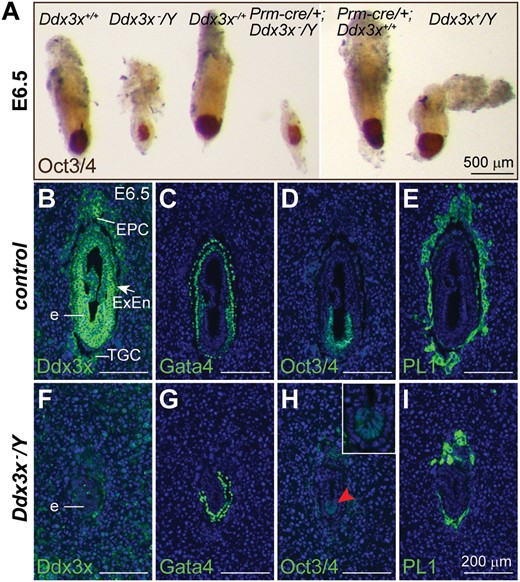
Loss of Ddx3x results in early embryonic lethality. (A) Whole-mount morphology of Ddx3x−/Y mutants and their littermates at E6.5. Representative E6.5 embryos with the indicated genotypes from the mating between Prm-cre/+;Ddx3x+/− female and Ddx3x+/Y male were immunostained with an antibody against Oct3/4 (brown). The Ddx3x−/Y mutants have a severely retarded growth compared with their littermate controls. (B−I) Immunohistochemical analysis of E6.5 embryos. Antibody against Ddx3x confirmed that Ddx3x protein was absent in Ddx3x−/Y embryos (F). Expression of GATA4, Oct3/4 and PL1 in adjacent sections revealed the presence of parietal and visceral endoderm, epiblast and trophoblast cell lineages, respectively, in littermate control and Ddx3x−/Y embryos. Higher magnification of epiblast (red arrowhead) in H is shown in the inset. Nuclei were stained with DAPI (blue). e, embryo; EPC, ectoplacental cone; ExEn, extraembryonic endoderm; TGC, trophoblast giant cell. Scale bars, 500 μm (A); 200 μm (B−I).
Ddx3x−/+ mutant with a maternal null allele exhibits placental defects
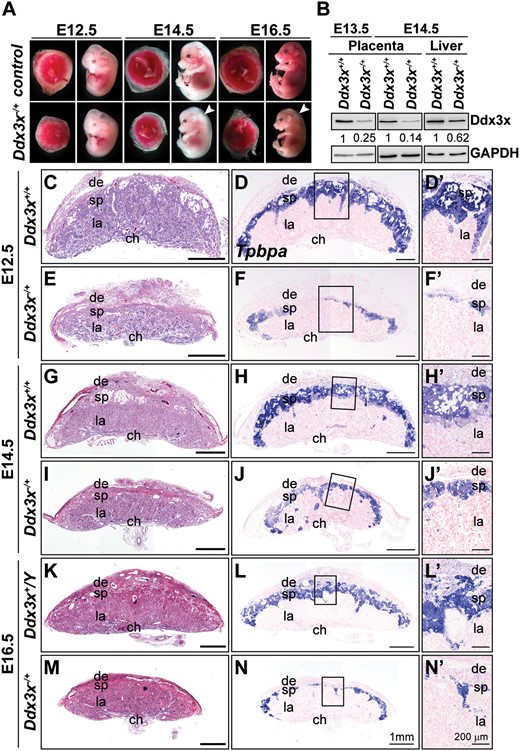
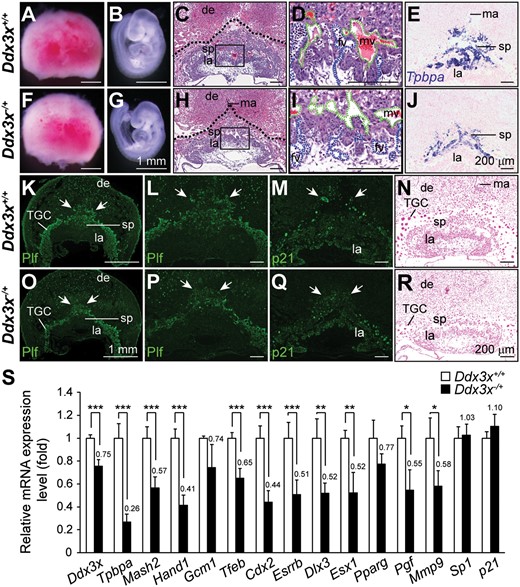
Targeted disruption of maternal Ddx3x allele causes placental defects and prenatal lethality in mice. (A) Whole-mount analysis of F2 Ddx3x−/+ mutant embryos and placentas. Representative images of wild-type littermates (Ddx3x+/+ and/or Ddx3x+/Y) and viable Ddx3x−/+ mutants at E12.5, E14.5, and E16.5. White arrowheads indicate whole body edema in Ddx3x−/+ mutants. (B) Ddx3x expression in placentas and livers from Ddx3x+/+ and Ddx3x−/+ embryos. Tissue extracts prepared from E13.5 and E14.5 Ddx3x+/+ littermates revealed the expected 72 kDa band, whereas Ddx3x expression levels in Ddx3x−/+ placentas were significantly reduced. GAPDH was used as the loading control. Relative Ddx3x protein levels were quantified using ImageJ software. (C−N’) Reduced thickness of spongiotrophoblast layers in mutant Ddx3x−/+ placentas. Hematoxylin and eosin (H&E)-stained midline placental sections from E12.5, E14.5, and E16.5 wild-type (C, G, K) and Ddx3x+/−(E, I, M) embryos. Placental size and spongiotrophoblast layer thickness are drastically reduced in the Ddx3x−/+ mutants compared with those of controls. RNA in situ hybridization analysis of a Tpbpa probe (blue) marks the spongiotrophoblast layer of placentas from wild-type (D, H, L) and Ddx3x−/+ mutants (F, J, N). A significant reduction in Tpbpa-expressing cells occurs in placentas from Ddx3x−/+ embryos. Higher magnification of boxed areas in D, F, H, J, L, and N are shown in D’, F’, H’, J’, L’, and N’, respectively. Nuclei were counterstained with nuclear fast red. ch, chorion; de, decidua; la, labyrinth; sp, spongiotrophoblast. Scale bars, 1 mm (C−N); 200 μm (D’−N’).
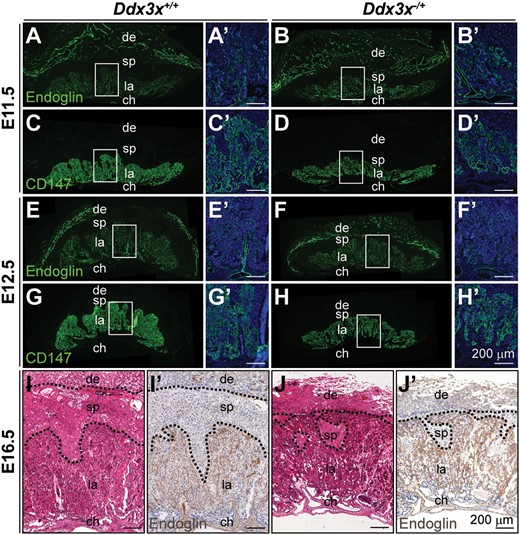
Abnormal labyrinth layer in Ddx3x−/+ placenta. Endoglin- (A−B’, E−F’, I’ and J’), CD147- (C−D’ and G−H’) and H&E- (I and J) stained placental sections from E11.5, E12.5, and E16.5 embryos. In placentas from Ddx3x+/+ controls, the labyrinth layers were well-developed revealed by endoglin-positive endothelial cells (A, E and I’) and CD147-positive trophoblasts (C and G), as gestation progressed. Placentas from Ddx3x−/+ embryos (B, D, F, H and J’) were underdeveloped with thinner and less organized labyrinth layers. Boxed areas in A−H are shown at higher magnification in A’−H’, respectively. The nuclei were counterstained with DAPI. ch, chorion, de, decidua; la, labyrinth; sp, spongiotrophoblast. Scale bar, 200 μm.
Paternal XCI of Ddx3x in mouse extraembryonic tissues affects trophoblast development
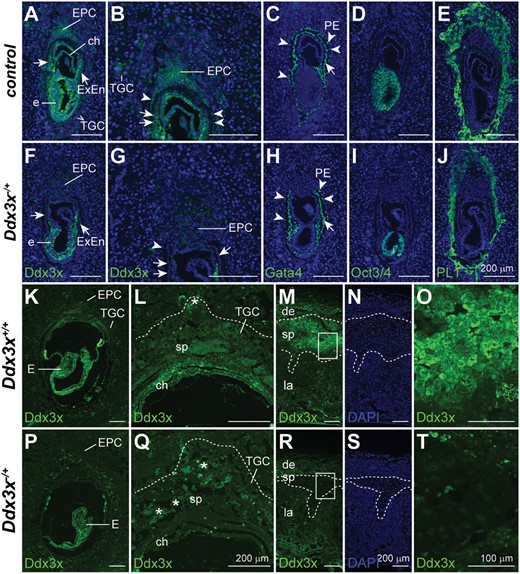
Paternally derived Ddx3x is preferentially inactivated in extraembryonic tissues of Ddx3x−/+ female embryos. Immunofluorescence analyses of Ddx3x, Gata4, Oct3/4, and PL1 expression in serial sections of E7.5 implantation sites. High Ddx3x levels were detected in embryos and extraembryonic tissues (EPC, chorion, extraembryonic endoderm, and parietal endoderm) in controls. Weak Ddx3x expression was observed in TGCs (A). Ddx3x levels were moderately reduced in Ddx3x−/+ embryo proper (F), whereas Ddx3x was significantly reduced in extraembryonic tissues of Ddx3x−/+ embryos compared with those of Ddx3x+/+ littermates (A). Higher magnification of E7.5 EPC areas in A and F are shown in (B) and (G), respectively. Gata4 expression was used to identify the visceral endoderm (arrows) and parietal endoderm (arrowhead) in control (C) and Ddx3x−/+ (H) embryos. Anti-Oct3/4 antibody specifically stained the epiblasts of control (D) and Ddx3x−/+ (I) embryos. PL1-positive trophoblast cells in Ddx3x−/+ implantation site (J) were less protrusive than those in littermate control (E). Nuclei were stained with DAPI (blue). Immunohistochemical analysis of Ddx3x in E8.5 implantation sites (K, L, P and Q) and E16.5 placentas (M−O and R−T) from Ddx3x+/+ and Ddx3x−/+ embryos. The expression of Ddx3x is relative higher in embryo than in the extraembryonic tissues (K). In Ddx3x−/+, the levels of Ddx3x in embryo was slightly lower than the littermate control, however, the protein was uniformly distributed in all embryonic cells (P). Ddx3x is moderately expressed in EPC, while weak expression was observed in TGCs in Ddx3x+/+ implantation site (K). In the Ddx3x−/+ extraembryonic tissues, the expression of Ddx3x was significantly reduced, specifically in EPC (P and Q). Higher magnification of EPC regions in K and P are shown in L and Q, respectively. Dotted lines demarcate the outer border of TGC layer. Asterisks indicate the autofluorescence from blood cells. At E16.5 placenta, Ddx3x expression was highest in spongiotrophoblast layer, albeit not at uniform levels in all cells. Expression levels of Ddx3x were very low, but detectable, in the decidua and labyrinth layer (M). Consistently, the expression of Ddx3x in the spongiotrophoblast layer was significantly reduced in Ddx3x−/+ placenta (R) compared with Ddx3x+/+ littermate (M). Dotted lines demarcate the boundary of spongiotrophoblast layer of the placenta. Boxed areas in M and R are shown at higher magnification in O and T, respectively. The nuclei were counterstained with DAPI (N and S). ch, chorion, de, decidua; E, embryo; EPC, ectoplacental cone; ExEn, extraembryonic endoderm; la, labyrinth; PE, parietal endoderm; sp, spongiotrophoblast; TGC, trophoblast giant cell. Scale bars, 200 μm (A−N, P−S); 100 μm (O, T).
Loss of Ddx3x in Ddx3x−/+ extraembryonic tissues leads to abnormal placental development and marker gene expression. Representative images of placentas and embryos from E9.5 Ddx3x+/+ littermates and Ddx3x−/+ mutants. Placentas from Ddx3x+/+ littermate (A) and Ddx3x−/+ mutant (F) were similar in size, whereas the Ddx3x−/+ embryo (G) was slightly smaller than that of the littermate (B). H&E stained placental sections from E9.5 Ddx3x−/+ mutant (H) showed relatively fewer spongiotrophoblast cells than that in littermate control (C). Dotted lines demarcate the outer placental surface. Boxed areas in C and H are shown at higher magnification in D and I, respectively. Branching morphogenesis of the chorioallantoic interface was apparent in placenta from Ddx3x+/+ control (D). Fetal vessels (filled with nucleated embryonic erythrocytes, blue dashed lines) were juxtaposed with maternal blood vessels (filled with enucleated erythrocytes, green dashed lines). Fetal vessels remained in the chorioallantoic plate region of mutant Ddx3x−/+ placenta (I). Tpbpa-expressing cells localized almost exclusively to the spongiotrophoblast layer of Ddx3x+/+ littermate placenta (E). An apparent reduction in Tpbpa-expressing cells was observed in Ddx3x−/+ placenta (J). Characterization of trophoblast cells in E9.5 placentas. The populations of Plf-positive primary and secondary TGCs (indicated by white arrows) were decreased in Ddx3x−/+ placenta (O) than those in littermate control (K). The distribution of outer TGC layer in Ddx3x−/+ placenta (arrows, P) was less protrusive than those in littermate control (L). The expression of p21 was detected in all three trophoblast layers of Ddx3x+/+ placenta: the outer TGC layer, spongiotrophoblast and labyrinth layer (M). The p21-positive nuclei (arrows) in secondary TGCs surrounding the spongiotrophoblast layer in Ddx3x−/+ placenta were smaller, while the expression of p21 in labyrinth layer was not altered (Q). Feulgen-stained placental sections showed the size of the nuclei and the staining intensities of TGCs were reduced in Ddx3x−/+ placenta (R) compared with the Ddx3x+/+ placenta (N). (S) Quantitative RT-PCR analysis of Ddx3x and marker genes in placentas from E9.5 Ddx3x+/+ and Ddx3x−/+ embryos. n = 7 per group. Error bars indicate SEM; *P < 0.05, **P< 0.01, ***P < 0.005. de, decidua; fv, fetal vessel; la, labyrinth; ma, maternal artery; mv, maternal vessel; sp, spongiotrophoblast; TGC, trophoblast giant cell. Scale bars, 1 mm (A, B, F, G, K, O); 200 μm (C−E, H−J, L−N, P−R).
To gain mechanistic insight into Ddx3x function during placental development, key transcription factors known to have important roles in trophoblast differentiation and placental development were analyzed (Fig. 5S). Reduced expression of Ddx3x and Tpbpa were first confirmed by quantitative RT-PCR (qRT-PCR) analysis of E9.5 Ddx3x−/+ placentas. The decreased expression of Ddx3x was detected in chorion, TGCs and EPC but not in the maternally derived decidua of E9.5 Ddx3x−/+ placenta (Supplementary Material, Figure S5), therefore, the expression level of Ddx3x was only decreased to 75% from that of Ddx3x+/+ controls. Mash2 is a crucial transcription factor for spongiotrophoblast proliferation and survival (22), whereas Hand1 is implicated in TGC differentiation (23). Gcm1 is essential for branching morphogenesis in chorioallantoic development (24). Tfeb is a bHLH-zipper transcription factor that is important for vessel branching within the labyrinth (25). Cdx2 is the earliest known transcription factor with a crucial role in trophoblast lineage cells (26). ERR-β (Esrrb) is a member of orphan nuclear receptor superfamily that is required for trophoblast proliferation and differentiation (27). Expression levels of all these transcription factors except Gcm1 were significantly reduced in E9.5 Ddx3x−/+ placentas. Previous studies report that Dlx3 is an important transcriptional regulator of placental labyrinth morphogenesis (28). We observed that Dlx3 expression was reduced in mutant Ddx3x−/+ placentas compared with those of their littermate controls. The expression of Dlx3 downstream targets, such as Esx1 (29), Pparg (30), Pgf (31) and Mmp9 (32) were consistently reduced in Ddx3x−/+ placentas; all these were significantly reduced except for Pparg.
Previous studies showed that Sp1-deficient mutants exhibit a broad range of phenotypic abnormalities and die around E10.5, indicating that Sp1 is essential during embryogenesis (33). Compound heterozygous Sp1/Sp3 mutant mice result in embryonic lethality and display a markedly reduced spongiotrophoblast layer and severe disorganization of the labyrinth layer in placenta, indicating that Sp1 and Sp3 act cooperatively to regulate downstream targets during embryo development (34). We previously reported that DDX3X interacted and cooperated with Sp1 to up-regulate expression of the p21 and inhibited colony formation in various human tumor cell lines and murine NIH3T3 cells (35). Nevertheless, expression levels of both Sp1 and p21 were unaffected in Ddx3x−/+ placentas (Fig. 5S). Together, these results indicate that paternal XCI in the extraembryonic tissues of Ddx3x−/+ mutants affects the trophoblast differentiation, which leads to aberrant placental layers (EPC and spongiotrophoblast) and defective vascularization in placental labyrinth. These placental abnormalities impair maternal blood supply to the embryo and ultimately lead to fetal growth restriction and lethality.
Ddx3x exhibits developmental- and tissue-specific differences in escape from XCI
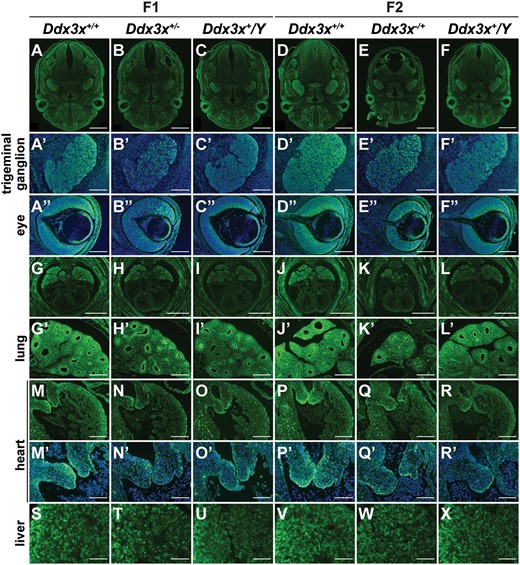
Ddx3x exhibits tissue-specific differences in escape from XCI. Immunohistochemical analysis of E14.5 embryonic tissues from F1 and F2 generations. Ddx3x is ubiquitously expressed in multiple cell types of different tissues and organs, including brain, trigeminal ganglion, eye, nasal cavity, lung, heart and liver, as visualized by anti-Ddx3x immunostaining. Higher Ddx3x expression levels in Ddx3x+/+ females (A, D, G and J) than in Ddx3x+/Y males (C, F, I and L) confirmed that Ddx3x escapes from XCI in females. Ddx3x protein levels in both heterozygous Ddx3x+/−(B and H) and Ddx3x−/+ (E and K) females were lower than their Ddx3x+/+ female littermates. The cells with very low or no detectable Ddx3x signal were randomly distributed in trigeminal ganglions and neural retina of eyes in Ddx3x+/−(B’ and B”) and Ddx3x−/+ (E’ and E”) females. Trigeminal ganglion and eye in A-F are shown at higher magnification in A’−F’ and A”−F”, respectively. Ddx3x was expressed at high level in the lung compared with the heart (G−L). We noted the heterogeneous expression of Ddx3x in the lung. Ddx3x were expressed at high levels mainly in the distal epithelium and mesenchyme (G’−L’). The expression levels and patterns of Ddx3x in Ddx3x+/−(H’) and Ddx3x−/+ (K’) lungs were comparable to those in the Ddx3x+/Y(I’ and L’) males. The relative levels of Ddx3x in the chamber endocardium and the cushion endocardium and mesenchymal cells were higher in the heart (M−R). As shown in mitral valves (M’−R’), the cells with very low Ddx3x signal were randomly distributed in Ddx3x+/−(N’) and Ddx3x−/+ (Q’) females. Lung and heart in G−L are shown at higher magnification in G’−L’ and M−R’, respectively. Mitral valves in M−R are shown at higher magnification in M’−R’. The expression of Ddx3x in the liver was heterogeneous and showed no significant differences in Ddx3x+/−(T) and Ddx3x−/+ (W) females compared with their littermates. The nuclei were counterstained with DAPI (blue). Scale bars, 1 mm (A−F, G−L); 200 μm (A’−F”, G’−R); 100 μm (M’−X).
Ddx3x is crucial for embryo survival
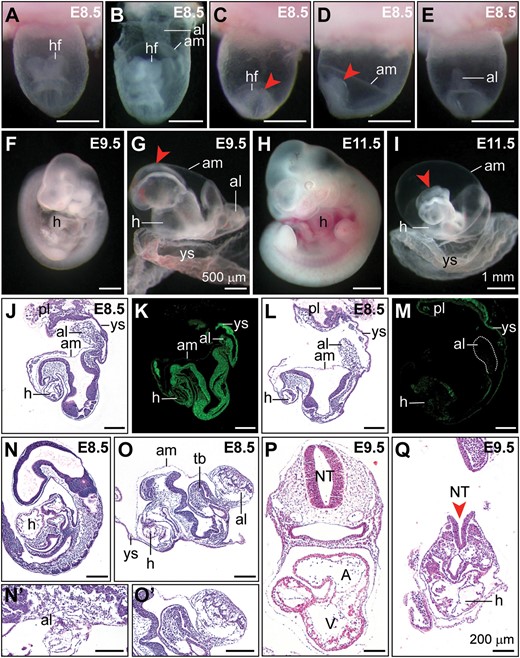
Developmental defects in Sox2-cre/+;Ddx3xflox/Y mutants. (A−I) Representative images of littermate controls and Sox2-cre/+;Ddx3xflox/Y mutants at E8.5, E9.5, and E11.5. Frontal view of the control embryo within the yolk sac (A) showed the normal appearance of the head region. In the early E8.5 control embryo (B) with the yolk sac partially dissected, the allantois was attached to the chorion. Abnormal headfolds (arrowheads) were observed in Sox2-cre/+;Ddx3xflox/Y mutants (C, frontal view; D, lateral view, anterior to the left). In Sox2-cre/+;Ddx3xflox/Y mutants (E, dorsal view), the allantois did not fuse to the chorion and appears as a bud. At E9.5, control embryo was dissected from the yolk sac and showed normal turned appearance (F). The Sox2-cre/+;Ddx3xflox/Y mutant displayed severe growth retardation, abnormal headfolds (arrowhead), and failure to turn (G). At E11.5, the allantois did not fuse to the chorion and appeared as an abnormal balloon-like shape in the Sox2-cre/+;Ddx3xflox/Y mutant (I). Defective neural tube closure (arrowhead) and strikingly disorganized posterior body were observed in the Sox2-cre/+;Ddx3xflox/Y mutant compared with those in the littermate control (H). H&E stained sagittal sections of E8.5 littermate control (J) and Sox2-cre/+;Ddx3xflox/Y mutant (L). Sox2-cre/+;Ddx3xflox/Y mutant exhibited a shorter allantois that had not expanded to make contact with the chorion. Immunohistochemical staining showed that Ddx3x protein was strongly localized in the embryo proper of the control (K), whereas very weak Ddx3x protein staining was observed in the Sox2-cre/+;Ddx3xflox/Y mutant (M). Histological sections of late-stage E8.5 littermate control (N) that had completed turning and established allantois contact with the chorion (N’). Sox2-cre/+;Ddx3xflox/Y mutant failed to undergo embryo turning, and displayed developmental delay with irregularly shaped tail bud and bulbous allantois (O). Higher magnification view of the allantois in O is shown in O’. H&E stained sections of E9.5 embryos revealed normal development in control embryo (P), whereas Sox2-cre/+;Ddx3xflox/Y mutant displayed growth retardation, defective neural tube closure (arrowhead), and cardiac malformation (Q). A, atrium; al, allantois; am, amnion; NT, neural tube; hf, headfold; h, heart; pl, placenta; tb, tail bud; V, ventricle; ys, yolk sac. Scale bars, 500 μm (A−G); 1 mm (H, I); 200 μm (J−Q).
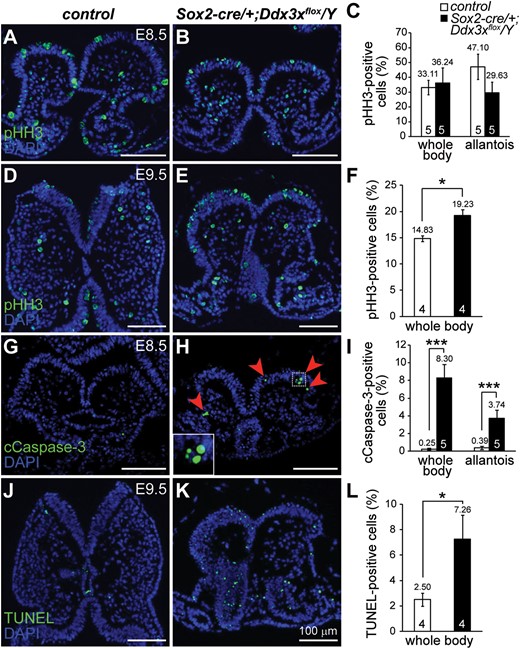
Increased mitotic index and apoptosis in Sox2-cre/+;Ddx3xflox/Y mutant embryos
Cell proliferation and cell apoptosis in Sox2-cre/+;Ddx3xflox/Y mutant embryos. Mitotic marker pHH3-positive cells in controls (A and D) and Sox2-cre/+;Ddx3xflox/Y mutants (B and E). At early E8.5 before embryo turning, there were no significant differences in pHH3-positive cells (green) between controls and mutants (A and B). The number of pHH3-positive cells was significantly higher in E9.5 Sox2-cre/+;Ddx3xflox/Y mutant (E) than in control (D). cCaspase-3- and TUNEL-positive cells in controls and Sox2-cre/+;Ddx3xflox/Y mutants. cCaspase-3-positive cells (green) were barely detectable in the E8.5 control (G), whereas the Sox2-cre/+;Ddx3xflox/Y mutant (H) contained more cCaspase-3-positive cells (arrowheads). Higher magnification view of the boxed area is shown in the inset. There was a significant increase in TUNEL-positive cells (green) in E9.5 Sox2-cre/+;Ddx3xflox/Y mutant (K) compared with that of the control (J). Nuclei were stained with DAPI (blue). Quantitative analysis of pHH3- (C and F), cCaspase-3- (I), and TUNEL-positive (L) cells in the whole body and allantois of controls and Sox2-cre/+;Ddx3xflox/Y mutants was determined by the percentage of positive cells with respect to total cells in each region, and presented as mean ± SEM. For each embryo, at least two adjacent sections were counted. n = 5 for E8.5 embryos and n = 4 for E9.5 embryos. *P < 0.05 and ***P < 0.005.
Deficient Ddx3x expression leads to DNA damage and cell cycle arrest during embryogenesis
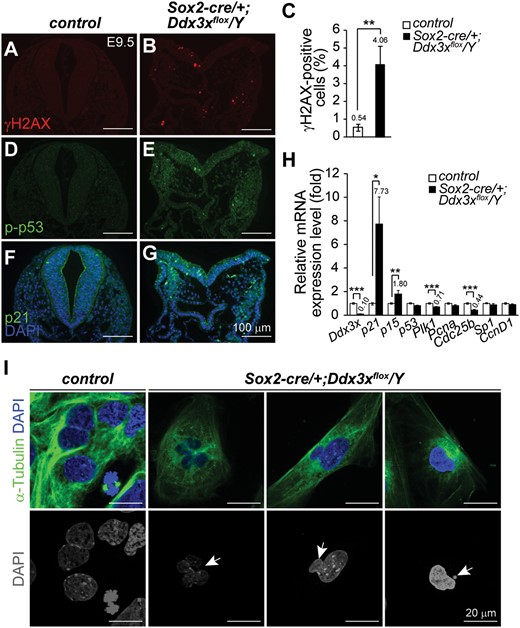
Increased DNA damage and nuclear abnormality in Sox2-cre/+;Ddx3xflox/Y mutants. (A−C) Representative γH2AX immunostaining in control and Sox2-cre/+;Ddx3xflox/Y mutant at E9.5. Significantly greater numbers of γH2AX-positive cells (red) were detected in Sox2-cre/+;Ddx3xflox/Y mutants than in controls (C). Percentages of γH2AX-positive cells in controls (n = 5) and Sox2-cre/+;Ddx3xflox/Y mutants (n = 6) were determined by the number of positive cells/total cells in each region and presented as mean±SEM. For each embryo, at least two adjacent sections were counted. (D−G) Expression levels of p21 and p-p53ser15 (green) were higher in Sox2-cre/+;Ddx3xflox/Y mutants than in littermate controls. Nuclei were counterstained with DAPI (blue). (H) Quantitative RT-PCR analysis of Ddx3x and cell cycle regulators in E9.5 controls and Sox2-cre/+;Ddx3xflox/Y mutants. *P < 0.05, **P < 0.01, ***P < 0.005. (I) Abnormal nuclei are observed in primary cells from Sox2-cre/+;Ddx3xflox/Y mutants. Disaggregated primary cells from E8.5 littermate controls and Sox2-cre/+;Ddx3xflox/Y embryos were immunostained with anti-α-Tubulin antibody and analyzed by confocal microscope (Olympus FV1000). Nuclei were visualized by DAPI staining. Merged images of α-Tubulin (green) and DAPI (blue) are shown in the upper panel, while the bottom panel show DAPI only (gray). Representative abnormal nuclei (arrows) were detected in primary cells from Sox2-cre/+;Ddx3xflox/Y mutants. Scale bars, 100 μm (A, B, D, E, F, G); 20 μm (I).
We next analyzed whether loss of Ddx3x affects the expression of several genes involved in cell cycle regulation in response to DNA damage by qRT-PCR (Fig. 9H). Ddx3x expression was significantly reduced in E9.5 Sox2-cre/+;Ddx3xflox/Y mutants as expected. The p21 level was elevated by 7.73-fold in Sox2-cre/+;Ddx3xflox/Y mutants compared with controls, and this result was consistent with that of the immunostaining study. The p15Ink4b (p15, Cdkn2b, Cdk inhibitor 2B) level was increased by 1.8-fold in the mutants. The p53 RNA level was reduced in the mutant compared with that of the control, despite the presence of p-p53ser15 signals in Sox2-cre/+;Ddx3xflox/Y mutants. The profound increases in p21 and p15 are implicated in halting cell cycle progression in response to DNA damage and mitotic defects (37). Polo-like kinase (Plk1) is an important component of mitosis, meiosis and cytokinesis levels (38). Proliferating cell nuclear antigen (Pcna) plays important roles in DNA replication and repair (39,40). Cell division cycle 25b (Cdc25b) phosphatase is a key component in normal cell cycle transition and G2/M DNA damage checkpoint (41,42). The Plk1 and Cdc25b were reduced significantly in Sox2-cre/+;Ddx3xflox/Y mutants, while Pcna showed the same trend of reduction. No changes in RNA levels of Sp1 and cyclin D1 (Ccnd1) were detected in Sox2-cre/+;Ddx3xflox/Y mutants. To further facilitate the study of Ddx3x cellular function in embryogenesis, we attempted to culture embryo explants from Sox2-cre/+;Ddx3xflox/Y mutants and their littermate controls. While primary cells from littermate controls grew well, cells from Sox2-cre/+;Ddx3xflox/Y mutants did not survive in culture dishes for long. Morphological abnormal nuclei and micronuclei were frequently observed in primary cells from Sox2-cre/+;Ddx3xflox/Y mutants, indicating loss of Ddx3x increased DNA damage and nuclear abnormalities, and triggered cell cycle arrest (Fig. 9I).
To evaluate the effect of Ddx3x on cell cycle progression, siRNA knockdown of Ddx3x expression in mouse C3H10T1/2 mesenchymal cells was performed. Cell proliferation was assessed using a metabolic MTS-based assay, which showed that proliferation was significantly reduced in siDdx3x knockdown cells compared with that in siCtrl control cells (Supplementary Material, Figure S9A and B). Propidium iodide staining and flow cytometry analysis indicated that the G2/M cell population showed an increasing trend in asynchronous siDdx3x cells compared with that in siCtrl cells at 96 h after siRNA treatment (Supplementary Material, Figure S9C). Furthermore, binuclear and γH2AX-positive cells were significantly higher in siDdx3x cells than in siCtrl cells (Supplementary Material, Figure S9D and E). These results prompted us to speculate that cell cycle progression was halted in response to DNA damage, and that G2/M population accumulated 96 h post-siDdx3x treatment.
To extend this result, siDdx3x and siCtrl cells were synchronized with a double thymidine (DT) block and then released at different time points and subjected to cell cycle analysis (Supplementary Material, Figure S9F). The results indicated that 60.3% and 39.7% of siCtrl cells were successfully synchronized at G0/G1 and S phases, respectively. After the release from DT block, siCtrl cells progressed normally through S and G2/M phases and reappeared as G0/G1 cells at 6 hours. In addition to 68.1% and 29.1% of siDdx3x cells were arrested at G0/G1 and S phases, respectively, we observed that 2.8% of siDdx3x cells were retained at G2/M phase after synchronization. After release from the DT block, siDdx3x cells progressed slowly from G0/G1 through S to G2/M and reappeared as G0/G1 cells at 12 h. Although siDdx3x cells initially displayed slow cell cycle progression, similar cell cycle phase distributions were observed in siCtrl and siDdx3x cells at 24 h after release from the DT block compared with their asynchronous cells. Kinetic changes in expression of cell cycle regulatory proteins after release from the DT block were analyzed by Western blotting (Supplementary Material, Figure S9G). The untreated basal levels of p21 and Plk1 were consistently higher and lower, respectively, in siDdx3x cells than in siCtrl cells. The p21 and Plk1 levels increased at 3 and 6 h, respectively, after release from the DT block in siCtrl and siDdx3x cells, and then gradually decreased to basal levels. Equivalent basal levels and kinetic changes in p53 and cyclin D1 levels were observed in siCtrl and siDdx3x cells. Together, these results indicated that Ddx3x functions as a positive cell cycle regulator. Ddx3x knockdown in C3H10T1/2 cells slowed cell cycles progression; however, cultured cells were probably not a physiologically relevant cell context for early embryonic cells, and allowed DNA repair and cell cycle progression. The current study emphasizes that Ddx3x has different effects in rapidly dividing embryonic cells and cultured mammalian cells, which have different cell cycle durations.
Discussion
Embryo development and growth depends on proper development of extraembryonic tissues, which connect the developing embryo to the mother’s uterine wall and enable the delivery of oxygen and nutrients to the fetus and the return of metabolic wastes from the fetus to maternal circulation. The work presented here demonstrates that a functional Ddx3x gene is essential for both extraembryonic and embryonic development in mice. We unraveled two distinct physiological functions of Ddx3x in vivo.
First, Ddx3x has an indispensable role in placental formation. Ddx3x+/− females heterozygous for a null Ddx3x allele of paternal origin are viable and fertile, whereas Ddx3x−/+ females with a maternal Ddx3x null allele do not survive. Epiblast-specific ablation of the maternal Ddx3x allele in Sox2-cre/+;Ddx3xflox/+ females can develop to viable adults confirmed that Ddx3x plays an essential developmental role in extraembryonic tissues. The paternal Ddx3x allele in extraembryonic female tissues is subjected to XCI during development. The absence of Ddx3x leads to abnormal trophoblast differentiation, which subsequently affects normal development of the placental labyrinth and maternal-fetal interface, and eventually results in the lethality of Ddx3x−/+ females. Although live Ddx3x−/+ females were harvested at E16.5−E18.5, some dead Ddx3x−/+ females can be obtained at E9.5 (Supplementary Material, Table S1). Phenotypic variability in mutant Ddx3x−/+ placenta was also observed in this study. A likely explanation could be that imprinted XCI in placenta tends to be partial and incomplete (43); thus, the residual Ddx3x protein could be different, which contributes to variable placental insufficiency among individuals. This study indicates that Ddx3x null allele cannot be transmitted from Ddx3x+/− females to both male and female offspring due to early embryonic lethality and paternal XCI in the extra-embryonic tissues, respectively. Early reports of a small number of X-link genes (G6PD, AR, PGK1 and HPRT) showed controversial XCI patterns in different human extraembryonic cell lineages and at different gestational ages. However, a more extensive analysis of 22 X-linked genes in full-term human placentas concludes that XCI occurs randomly (44). Although DDX3X shares 99% sequence identity among human and mouse, it is possible that DDX3X gene expression and function differs in human and mouse extraembryonic tissues. Thus, it is of clinical interest to know whether heterogeneous DDX3X expression in extraembryonic tissues is a genetic cause of pregnancy complication in a heterozygous human female who may carry a DDX3X null allele.
Second, Ddx3x disruption affects basic cell cycle regulation and cell viability of embryo proper during mouse development. In this study, we found that Ddx3x−/Y mutants can be recovered at E6.5, albeit at a lower frequency than expected. The average of 8.1 implantation sites per litter was obtained from Ddx3x+/− mutants, suggesting that embryonic lethality occurred at an early post-implantation stage. The levels of γH2AX and p-p53Ser15, which are indicators of DNA damage, were increased in Sox2-cre/+;Ddx3xflox/Y mutants. Loss of Ddx3x leads to significant increases in p21 and p15 expression levels. Cell cycle arrest in response to DNA damage is not well tolerated in rapidly growing cells in the absence of Ddx3x. Eventually, Sox2-cre/+;Ddx3xflox/Y mutants exhibit severe developmental delay and die around E11.5. Li et al. (45) reported that cytoplasmic microinjection of Ddx3x siRNA into zygotes or single blastomeres of 2-cell mouse embryos affected subsequent development into blastocysts in vitro. The reduced cell numbers and increases in p53 and apoptotic cells were observed in blastocysts treated with Ddx3x siRNA. Although early embryo growth in vitro can closely mimic growth in vivo, reciprocal interactions between embryo, extraembryonic compartments and receptive uterus are lacking in in vitro embryonic cultures. As development proceeds, the interaction between embryo and extraembryonic tissues orchestrates a burst of cell proliferation (fast embryonic cycles) and embryonic organization at the perigastrulation stage (46). The cell cycle machinery and progression of cultured embryonic cells described in the study of Li et al. (45) and the physiological condition of rapidly proliferating embryonic cells at the implantation site in the present work reveal a discrepancy between these two studies.
Our previous studies showed that DDX3X interacts and cooperates with Sp1 to transcriptionally activate the p21 gene (35,47). This study revealed a disparate relationship between Ddx3x and p21. We showed that Ddx3x is strongly expressed in embryonic and extraembryonic tissues derived from the inner cell mass, whereas relatively moderate Ddx3x levels are detected in the trophectoderm-derived EPC and TGCs (Figs 1 and 4). Conversely, p21 protein is readily detected in EPC and TGCs, but barely detectable in the early embryo proper (Fig. 5 and data not shown). Loss of Ddx3x in Sox2-cre/+;Ddx3xflox/Y embryos led to significant increases in both p21 mRNA and protein expression. However, we did not detect the alteration of p21 levels in E9.5 Ddx3x−/+ placentas lacking Ddx3x (Fig. 5S). The differential expression patterns of p21 and Ddx3x in these different physiological contexts implied that p21 and Ddx3x may coordinate differently with cell cycle machinery and regulatory networks to ensure precise cell proliferation (in proliferating embryonic cells) and post-implantation differentiation (in EPC and TGCs). The biasing factors contributing to discrepancies in all these results include different experimental conditions, different cellular contexts and cell model systems, and the relative levels of Ddx3x and p21 proteins.
Proper spatiotemporal execution of active cell proliferation and cell cycle arrest to repair damaged DNA is crucial to maintain genome integrity. Aberrant DDX3X expression has been implicated in tumorigenesis through the dysregulation of cell growth, cell cycle progression, and survival. Numerous studies, including ours, in different cell lines and patient samples revealed different roles of DDX3X in cell cycle and cell growth control, which generated some controversy in the literature (10,12,35,47–53). Controversial roles of DDX3X in cellular behavior and inconsistent results might be due to different experimental approaches in different cellular and physiological contexts.
Recent genome-wide studies reported that mutations in human DDX3X are associated with several important human diseases, including head and neck squamous cell carcinoma (54), gingivo-buccal oral squamous cell carcinoma (55), medulloblastomas (56–58), chronic lymphocytic leukemia (59), pediatric T-cell acute lymphoblastic leukemia (60), natural killer/T-cell lymphoma (61), developmental disorders (13) and ID (14). In this study, we found Ddx3x exhibits developmental- and tissue-specific differences in escape from XCI in both Ddx3x+/− and Ddx3x−/+ females. Further work is needed to verify how most of these mutations affect physiological functions and determine dose-dependent effects of DDX3X in these diseases. Knowledge of DDX3X dynamics in cell cycle regulation, cell cycle checkpoint, and DNA damage response will reveal how DDX3X spatiotemporally orchestrates these processes to protect and maintain cellular DNA integrity. A small molecule was designed to inhibit DDX3X activity, which consequently promoted tumor regression in lung cancer (53). Elucidating the precise functions of DDX3X in different cell lines and cell contexts will facilitate rational therapeutic designs and strategies to prevent and treat diseases and cancer in the future.
Materials and Methods
Mice
To generate the Ddx3x null allele, Ddx3xflox/flox mice were bred with the Prm-cre (Tg(Prm-cre)58Og/J) transgenic mouse line (16). Cre-mediated recombination resulted in deletion of the floxed Ddx3x sequence in the germline of compound heterozygous (Prm-cre/+;Ddx3xflox/Y) males. The Prm-cre/+;Ddx3xflox/Y males were then bred with wild-type females to obtain Ddx3x heterozygous (Ddx3x+/−) female mice. To generate epiblast-specific Ddx3x knockout mice, transgenic Sox2-cre males were mated with Ddx3xflox/flox females. Embryos and placentas from timed pregnant females were dissected under a stereomicroscope (Leica MZ6) and photographed using a DP20 digital camera (Olympus). The placenta is derived from both maternal and embryonic tissues. Because of the close contact between maternal and embryonic tissues, especially at E9.5, they were harvested as a whole placental unit. The experimental procedures using mice were approved by the Institutional Animal Care and Use Committee at National Yang-Ming University. Genotyping primer sets are listed in Supplementary Material.
Histology, immunohistochemistry, and RNA in situ hybridization
Histology and immunohistochemistry were performed as described previously in (62). The Feulgen reaction was performed on placental sections using DNA staining kit according to Feulgen (Merck). The antibodies used in this study are listed in Supplementary Material. Non-radioactive in situ hybridization on paraffin sections was performed using digoxigenin-labeled antisense mRNA probes for Tpbpa (nucleotide 475 − 1,295 of NM_009411), which were prepared according to the manufacturer’s instructions (Roche). After hybridization, probes were detected using alkaline phosphatase−conjugated anti-digoxigenin antibody and visualized with NBT/BCIP colorimetric detection (Roche). Images were acquired using the Axio Imager light microscope with AxioCam HRc (Carl Zeiss).
Whole-mount immunohistochemistry
Whole-mount immunostaining was performed as described previously in (56) with anti-Oct3/4 (Sigma) antibody. For signal amplification, embryos were incubated with biotinylated secondary antibody followed by reaction with horseradish peroxidase-conjugated streptavidin. The signal was detected using 3,3′-diaminobenzidine as a chromogen (Vector Laboratories).
TUNEL analysis
The DNA fragments of apoptotic cells in embryonic tissue sections were detected using the Fluorescein FragEL DNA Fragmentation Detection Kit (Merck Millipore) according to the manufacturer’s instructions.
RNA isolation and quantitative reverse transcription PCR analysis
RNA samples from mouse embryo and placenta were isolated and subjected to qRT-PCR analysis as described previously in (56). Gene expression was normalized with respect to Hprt expression. Student’s t-test was used to determine P values. The primer sequences are listed in Supplementary Material.
Supplementary Material
Supplementary Material is available at HMG online.
Acknowledgements
We thank Wan-Chun Yu and Yu-Hong Tsai for technical assistance in generating Ddx3x targeting construct; Dr Ming-Ta Hsu for constant advice and support; Dr Sophia Y. Tsai and Dr Ming-Jer Tsai for helpful discussions and for critically reading the article. We are grateful to ‘Transgenic Mouse Models Core of the National Core Facility Program for Biotechnology, Ministry of Science and technology’ and the ‘Gene Knockout Mouse Core Laboratory of National Taiwan University Center of Genomic Medicine’ for providing ES cell targeting and Chimera production services.
Conflict of Interest statement. None declared.
Funding
This work was supported by the Ministry of Science and Technology of Taiwan grant (NSC100-2320-B-009-007-MY3, MOST103-2320-B-009-006- and MOST104-2320-B-009-001- to Y.-H.W.L., NSC102-2320-B-010-023-MY3, MOST103-2320-B-010-029- and MOST104-2320-B-010-002- to L.-R.Y.); and the Ministry of Education, Aim for the Top University Plan grant (102AC-T505, 103AC-T603, and 104AC-T503 to L.-R.Y.).
References
Author notes
†The authors wish it to be known that, in their opinion, the first two authors should be regarded as joint First Authors.