-
PDF
- Split View
-
Views
-
Cite
Cite
Olof Stephansson, Henrik Falconer, Jonas F. Ludvigsson, Risk of endometriosis in 11 000 women with celiac disease, Human Reproduction, Volume 26, Issue 10, October 2011, Pages 2896–2901, https://doi.org/10.1093/humrep/der263
Close - Share Icon Share
Abstract
Endometriosis is a common cause of infertility. Whereas celiac disease (CD) is present in ∼1% of individuals in Western Europe, the prevalence in women undergoing investigation for infertility is often >2%. Still, the relationship between CD and endometriosis is unclear.
We identified 11 097 women with CD (Marsh 3: villous atrophy) through biopsy data from all 28 pathology departments in Sweden. Biopsies had been performed between 1973 and 2008. Data on inpatient and outpatient diagnoses of endometriosis were retrieved from the National Patient Register. We then used the Cox regression to estimate the hazard ratios (HRs) for endometriosis in women with CD to compare with those in 54 992 age-matched control women.
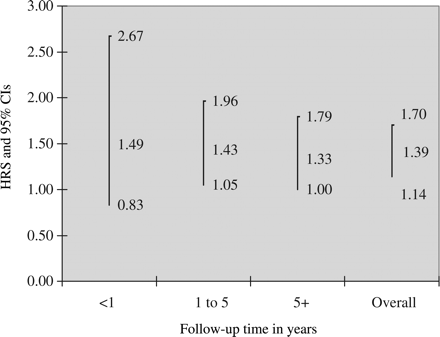
During the follow-up, 118 individuals with CD and 399 matched controls developed endometriosis. Hence, patients with CD were at increased risk of subsequent endometriosis [HR = 1.39; 95% confidence interval (CI) = 1.14–1.70]. The absolute risk of endometriosis in patients with CD was 112/100 000 person-years with an excess risk of 31/100 000. Risk estimates were highest in the first year after diagnosis (HR = 1.49; 95% CI = 0.83–2.67) and gradually decreased (>5 years after CD diagnosis, HR = 1.33; 95% CI = 1.00–1.79).
Endometriosis seems to be associated with prior CD. Potential explanations include shared etiological factors and CD-mediated inflammation.
Introduction
Celiac disease (CD) is a lifelong immune-mediated disorder in which patients demonstrate villous atrophy (VA), inflammation and crypt hyperplasia in the small intestine (Dickson et al., 2006). It has become evident in recent years that CD is associated with a number of extra-gastrointestinal symptoms, including depression (Ludvigsson et al., 2007), osteoporosis (West et al., 2003) and certain types of malignancy (Gao et al., 2009). Whereas CD is present in ∼1% of individuals in Western Europe (Green and Jabri, 2003; Dube et al., 2005; Walker et al., 2010), the prevalence in women undergoing investigation for infertility is often over 2% (Collin et al., 1996; Kolho et al., 1999; Meloni et al., 1999; Gasbarrini et al., 2000; Tiboni et al., 2006).
Endometriosis is a common gynecological disease with an estimated prevalence of 10% (Zhao et al., 1998). The main symptoms include dysmenorrhea, dyspareunia, irregular bleedings and chronic pelvic pain. Endometriosis is a common finding in women with infertility (from 20% to 50% of all women with infertility) and is associated with a lower pregnancy rate, not only from natural ovulatory cycles, but also after intrauterine insemination or in vitro fertilization and embryo transfer (Barnhart et al., 2002). The etiology of endometriosis is not completely known, although retrograde menstruation remains the most widely accepted pathogenic factor (Kennedy et al., 2005). Endometriosis meets many of the classification criteria for autoimmune disease, including T- and B-cell abnormalities, altered apoptosis, tissue damage and familial occurrence (Nothnick, 2001; Matarese et al., 2003). A recent study demonstrated an association between endometriosis and HLA-DRB1 (Sundqvist et al., 2010). Furthermore, the finding of both local and systemic inflammation (Pizzo et al., 2002) suggests that endometriosis may be classified together with chronic inflammatory diseases, such as rheumatoid arthritis and Crohn's disease.
An Italian study by Tiboni et al. (2006) found a non-significantly increased risk of CD in women undergoing assisted reproductive technology. To our knowledge, there exists only one study on the association between endometriosis and CD (Aguiar et al., 2009). In that study, Aguiar et al. screened 120 women with endometriosis and 1500 controls with CD serology followed by a small intestinal biopsy where indicated. CD was confirmed in 3/120 (2.5%) cases with endometriosis and in 10/1500 (0.66%) controls. This corresponded to an odds ratio of 3.8 but because of a lack of statistical power, the difference was not statistically significant (Fisher's exact test recalculated, P = 0.066).
The lack of large-scale investigations necessitated further studies on the possible association between CD and endometriosis. This study was designed to estimate the risk of developing endometriosis for women who had been diagnosed with CD. We did so by calculating absolute and relative risks for endometriosis in 11 097 women with biopsy-verified CD and 54 992 age-matched control women.
Materials and Methods
Using the personal identity number assigned to each Swedish resident (Ludvigsson et al., 2009a,b,c), we matched nationwide data on small intestinal biopsies with the Swedish National Patient Register (NPR, containing both inpatient and hospital outpatient care) to examine the risk of endometriosis. In all analyses, CD is the exposure and endometriosis the outcome measure.
Collection of biopsy data
The exposure of this cohort study was CD defined as small intestinal biopsy with VA. We originally collected data on 351 403 biopsy reports representing 287 586 unique individuals (176 097 women). Details on the collection of the biopsy data care are given below.
Villous atrophy
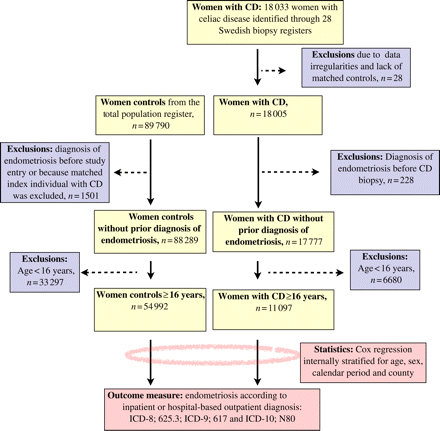
We used computerized biopsy reports from all Swedish pathology departments (n = 28) to identify patients with CD. Our data collection took place between October 2006 and February 2008; however, the biopsies per se had been carried out from 1969 to 2008. In total, we identified 18 033 women with CD. CD was defined in this study as having a SnoMed pathology code equal to VA (see the Appendix for a list of Swedish SnoMed codes translated into the international histopathology grading system by Marsh).
Controls
We sent the records of all patients with CD to the government agency, Statistics Sweden, for matching. Each index individual was matched with up to five controls on age, county and calendar period from the Total Population Register (Johannesson, 2002).
After matching and exclusion of data irregularities (Ludvigsson et al., 2009a,b,c), we had data on 18 005 women with CD and 89 790 female controls matched for age, calendar period and county (Fig. 1). We then excluded women with endometriosis before the biopsy or corresponding date in the matched controls. Because all analyses were performed per stratum, we also excluded controls whose index individual had been excluded for any reason. Our sample then consisted of 17 777 women with CD and 88 289 age-matched control women. None of these women had a diagnosis of endometriosis before study entry.
Since premenarchal immortal person-time may hide a true association between CD and endometriosis, we then restricted our data to women aged 16 or more at diagnosis of CD (and at study entry for reference individuals). We chose 16 years as our cut-off for study entry, since an earlier study (Herman-Giddens et al., 1997) has shown that more than 95% of white females are likely to have had their menarche at this age. The current study was therefore based on 11 097 women with a diagnosis of CD and 54 992 age-matched control women. As opposed to the whole biopsy cohort, all individuals in this study had been biopsied after year 1973.
Outcome measure
We defined endometriosis according to relevant ICD (international classification of disease) codes in the Swedish NPR (ICD-8, 625.3; ICD-9, 617 and ICD-10, N80; Fig. 1). In a previous study, 81% of patients with a diagnosis of endometriosis in the NPR were histologically confirmed (Melin et al., 2006). The ICD codes do not carry any information about disease severity.
Statistical analyses
We used an internally stratified Cox regression model to estimate hazard ratios (HRs) for endometriosis. The internal stratification means that the index individual was only compared with his or her controls within the same stratum and then a summary risk estimate was calculated. The proportional hazard assumption was tested by plotting log-minus-log curves. Follow-up started on the date of first biopsy with VA and the corresponding date in matched controls and ended with endometriosis diagnosis, emigration, death or on 31 December 2008, whichever event occurred first.
In predefined subanalyses, we studied the risk of endometriosis by the follow-up period (<1, 1 to <5 and ≥5 years), age (16–30, 31–45, 46–60 and ≥61 years at first biopsy) and calendar year of first biopsy (1973–1989, 1990–1999 and 2000–2008). We calculated incidence rates as the number of first recorded endometriosis diagnoses divided by person-years until first diagnosis or end of follow-up. To increase the specificity of endometriosis, we examined the risk of having an endometriosis diagnosis on at least two separate occasions. In a post hoc analysis, we also restricted our data set to women of reproductive age at study entry (16–45 years).
We used SPSS version 18.0 to perform all analyses. HRs with 95% confidence intervals (CIs) not including 1 were regarded as statistically significant.
Ethics
The study was approved by the Research Ethics Committee of Karolinska Institutet.
Results
The median age at CD diagnosis was 46 years and the median follow-up was 8 years. Women with CD had been biopsied between 1973 and 2008 (with a median year of biopsy of 1999). Roughly half of the women with CD had undergone their first biopsy in year 2000 or later. During the follow-up, 118 individuals with CD and 399 matched controls had a subsequent diagnosis of endometriosis.
CD and subsequent endometriosis
Individuals with CD were at an increased risk of endometriosis (HR = 1.39; 95% CI = 1.14–1.70). The absolute risk of endometriosis was 112/100 000 person-years in women with CD with an excess risk of 31/100 000 person-years. The percentage of endometriosis in patients with CD that may be related to CD was 28%. HRs for endometriosis decreased marginally with follow-up time (Fig. 2). Risk estimates were similar in the four age strata (interaction test between age at diagnosis and risk of endometriosis, P = 0.800), and there were no statistically significant differences in risk estimates for endometriosis according to calendar periods (P for interaction = 0.991).
When we restricted our outcome to having at least two diagnoses of endometriosis, the HR remained statistically significantly increased (HR = 1.49; 95% CI = 1.02–2.18).
In a post hoc analysis, we restricted our data set to women of reproductive age at study entry (16–45 years); they were at a 35% increased risk of later endometriosis (95% CI = 1.07–1.69).
Discussion
In this nationwide population-based study, we found a positive association between CD and endometriosis. The highest risk of subsequent endometriosis was found in the first year after CD diagnosis, i.e. before a gluten-free diet is likely to have had an effect on the small intestinal VA and inflammation.
Recently, Brazilian researchers reported a positive association between CD and endometriosis in a sample of 200 women (Aguiar et al., 2009). The prevalence of biopsy-verified CD in women with endometriosis was 2.5%, whereas the proportion of women with positive tissue transglutaminase was considerably higher (7.5%; Aguiar et al., 2009). In contrast to the Brazilian study, we were able to investigate the relationship between CD and endometriosis in a nationwide sample of more than 11 000 patients with CD and 54 000 matched controls. During the follow-up, 118 individuals with CD and 399 matched controls developed endometriosis, corresponding to an HR of 1.39.
Shared etiology and ongoing inflammation are candidate explanations for the positive association between CD and endometriosis. HLA-associated risk factors in CD include HLA-DQ2.5 or DQ8+ though HLA-DQ7 (DQA1*0505/DQB1*0301) may also influence the risk of future CD (in DQ2.5+ individuals; Monsuur et al., 2008). These HLA-related risk factors may be of importance since DQ7 is twice as common in patients with endometriosis than in patients without endometriosis (Ishii et al., 2003).
Recent data suggest that endometriosis is associated with chronic inflammation. This observation is of interest since inflammation in CD often persists for many years after diagnosis (despite the introduction of a gluten-free diet; Lee et al., 2003; Hopper et al., 2008). In the study by Lee et al. (2003), four out of five patients with CD showed mucosal abnormalities, including inflammation even 8 years after diagnosis and institution of a gluten-free diet. Not only could increased levels of inflammation in CD trigger endometriosis but it is also possible that the two diseases share certain expressions of inflammation. Major increases in interferon-γ and interleukin-6 are seen in both CD (Schuppan et al., 2009) and endometriosis (Othman Eel et al., 2008).
We cannot rule out the possibility that part of the increased risk of endometriosis is due to surveillance bias. Some patients with, for example, abdominal pain that is due to endometriosis may undergo investigation for CD. Still, ascertainment bias is unlikely to explain all of the risk increase in patients with CD since there remained a 33% increased risk of endometriosis even more than 5 years after biopsy. However, because of insufficient power, that risk increase was not statistically significant (Fig. 2; P = 0.054).
A major strength of our study includes the population-based design with biopsy-verified CD diagnosis. By using biopsy reports from all 28 Swedish pathology departments, we were able to identify more than 11 000 women with CD. The large numbers and excellent statistical power allowed for important subanalyses. It has been suggested that with an increasing use of CD serology, patients with milder forms of CD will be identified and that their risk of complications is lower than that of older generations of CD patients. We could not confirm this hypothesis since risk estimates for endometriosis were similar over the study period (P for interaction between CD and calendar period = 0.991).
We used biopsy reports with small intestinal VA to identify patients with CD. In a validation study, 95% of patients with VA also had CD (Ludvigsson et al., 2009a,b,c). This positive predictive value is actually higher than a physician-made diagnosis from the national inpatient register (Smedby et al., 2005). Although we did not request a positive CD serology for the diagnosis of CD, in a subset of randomly selected patients, 88% with available data on CD serology had positive antibodies at the time of biopsy (Smedby et al., 2005). Finally, biopsy reports have high sensitivity for diagnosed CD. Some 96% of interviewed Swedish gastroenterologists and 100% of interviewed pediatricians perform a small intestinal biopsy before diagnosing CD (Ludvigsson et al., 2009a,b,c).
A possible limitation of this study is our lack of histological verification of the endometriosis diagnosis. However, in a validation study, 81% of the women with a diagnosis of endometriosis were histologically confirmed (Melin et al., 2006). Another limitation is that we could not differentiate between women with CD adhering to a gluten-free diet and those with low adherence. In a subset of patients with CD in our cohort, 17% showed indications of low-dietary adherence; Ludvigsson et al., 2009a,b,c). It is possible that the risk of endometriosis in CD is confined to individuals with a low-dietary adherence. We also had no data on smoking and BMI in study participants. The role of smoking in endometriosis (Moen and Schei, 1997; Calhaz-Jorge et al., 2004; Chapron et al., 2010) and CD (Snook et al., 1996; Ludvigsson et al., 2005) is still unclear. While CD seems to be associated with lower BMI (Olen et al., 2009), endometriosis has been linked to both high (Calhaz-Jorge et al., 2004) and low (Ferrero et al., 2005) BMI. We cannot rule out that smoking and BMI have influenced our risk estimates.
Conclusion
Endometriosis seems to be associated with prior CD. Potential explanations include shared etiological factors and CD-mediated inflammation.
Authors' roles
ICMJE criteria for authorship read and met: J.F.L., O.S. and H.F; agree with the manuscript's results and conclusions: J.F.L., O.S. and H.F.; designed the experiments/the study: J.F.L.; analyzed the data: J.F.L.; collected data/did experiments for the study: J.F.L.; wrote the first draft of the paper: J.F.L.; contributed to the writing of the paper: O.S. and H.F.; contributed to design of study and interpretation of the data analyses and gave guidance on development of statistical models: J.F.L., O.S. and H.F.; interpretation of data and approved the final version of the manuscript: J.F.L., O.S. and H.F.; responsible for data integrity: J.F.L.; obtained funding: J.F.L.
Funding
O.S. was supported by a post-doctoral scholarship from the Swedish Society of Medicine and J.F.L. was supported by a grant from the Örebro University Hospital while writing this article. This project was supported by grants from The Swedish Society of Medicine, the Swedish Research Council–Medicine (522-2A09-195), the Sven Jerring Foundation, the Örebro Society of Medicine, the Karolinska Institutet, the Clas Groschinsky Foundation, the Juhlin Foundation, the Majblomman Foundation, the Uppsala-Örebro Regional Research Council and the Swedish Celiac Society.
References
Appendix
Table A1 A comparison of small intestinal histopathology classifications.
| Classification used in this project | VA | ||
| Marsh classification | Type IIIa | Type IIIb | Type IIIc |
| Marsh description | Flat destructive | ||
| Corazza et al.a | Grade B1 | Grade B2 | |
| SnoMed codes | M58, D6218, M58005 | M58, D6218, M58006 | M58, D6218, M58007 |
| KVAST/Alexander classification | III Partial VA | IV Subtotal VA | IV Total VA |
| Characteristics | |||
| VA | + | ++ | ++ |
| IEL# | + | + | + |
| Crypt hyperplasia | + | ++ | ++ |
| Classification used in this project | VA | ||
| Marsh classification | Type IIIa | Type IIIb | Type IIIc |
| Marsh description | Flat destructive | ||
| Corazza et al.a | Grade B1 | Grade B2 | |
| SnoMed codes | M58, D6218, M58005 | M58, D6218, M58006 | M58, D6218, M58007 |
| KVAST/Alexander classification | III Partial VA | IV Subtotal VA | IV Total VA |
| Characteristics | |||
| VA | + | ++ | ++ |
| IEL# | + | + | + |
| Crypt hyperplasia | + | ++ | ++ |
| Classification used in this project | VA | ||
| Marsh classification | Type IIIa | Type IIIb | Type IIIc |
| Marsh description | Flat destructive | ||
| Corazza et al.a | Grade B1 | Grade B2 | |
| SnoMed codes | M58, D6218, M58005 | M58, D6218, M58006 | M58, D6218, M58007 |
| KVAST/Alexander classification | III Partial VA | IV Subtotal VA | IV Total VA |
| Characteristics | |||
| VA | + | ++ | ++ |
| IEL# | + | + | + |
| Crypt hyperplasia | + | ++ | ++ |
| Classification used in this project | VA | ||
| Marsh classification | Type IIIa | Type IIIb | Type IIIc |
| Marsh description | Flat destructive | ||
| Corazza et al.a | Grade B1 | Grade B2 | |
| SnoMed codes | M58, D6218, M58005 | M58, D6218, M58006 | M58, D6218, M58007 |
| KVAST/Alexander classification | III Partial VA | IV Subtotal VA | IV Total VA |
| Characteristics | |||
| VA | + | ++ | ++ |
| IEL# | + | + | + |
| Crypt hyperplasia | + | ++ | ++ |