-
PDF
- Split View
-
Views
-
Cite
Cite
Allan A Pacey, Guido Pennings, Edgar Mocanu, Janne Rothmar, Anja Pinborg, Stine Willum Adrian, Corey Burke, Anne-Bine Skytte, An analysis of the outcome of 11 712 men applying to be sperm donors in Denmark and the USA, Human Reproduction, Volume 38, Issue 3, March 2023, Pages 352–358, https://doi.org/10.1093/humrep/deac264
Close - Share Icon Share
Abstract
Is the outcome of donor recruitment influenced by the country in which recruitment took place or the initial identity (ID)-release choice of applicants?
More applicants are accepted as donors in Denmark than in the USA and those who choose ID release are more frequently accepted than those who do not.
The successful recruitment of sperm donors is essential to provide a range of medically assisted reproduction (MAR) procedures, which rely upon donor sperm. However, while much has been written about the medical screening and assessment of sperm donors from a safety perspective, relatively little has been written about the process of recruiting donors and how it works in practice. There are differences in demographic characteristics between donors who choose to allow their identity to be released to their donor offspring (ID release) compared to those who do not (non-ID release). These characteristics may also influence the likelihood of them being recruited.
A total of 11 712 men applied to be sperm donors at a sperm bank in Denmark and the USA during 2018 and 2019.
Anonymized records of all donor applicants were examined to assess the number passing through (or lost) at each stage of the recruitment process. Statistical analysis was carried out to examine differences between location (Denmark or USA) and/or donor type (ID release versus non-ID release).
Few applicants (3.79%) were accepted as donors and had samples frozen and released for use; this was higher in Denmark (6.53%) than in the USA (1.03%) (χ2 = 243.2; 1 degree of freedom (df); z = 15.60; P < 0.0001) and was higher in donors who opted at the outset to be ID release (4.70%) compared to those who did not (3.15%) (χ2 = 18.51; 1 df; z = 4.303; P < 0.0001). Most candidate donors were lost during recruitment because they: withdrew, failed to respond, did not attend an appointment, or did not return a questionnaire (54.91%); reported a disqualifying health issue or failed a screening test (17.41%); did not meet the eligibility criteria at the outset (11.71%); or did not have >5 × 106 motile sperm/ml in their post-thaw samples (11.20%). At each stage, there were statistically significant differences between countries and the donor’s initial ID choice. During recruitment, some donors decided to change ID type. There were no country differences in the frequency in which this occurred (χ2 = 0.2852; 1 df; z = 0.5340; P = 0.5933), but it was more common for donors to change from non-ID release to ID release (27.19%) than the other way around (11.45%) (χ2 = 17.75; 1 df; z = 4.213; P < 0.0001), although movements in both directions did occur in both countries.
No information was available about the demographic characteristics of the applicants, which may also have influenced their chances of being accepted as a donor (e.g. ethnicity and age). Donor recruitment procedures may differ in other locations according to local laws or guidelines.
A better understanding of when and why candidate donors are lost in the recruitment process may help develop leaner and more efficient pathways for interested donors and sperm banks. This could ultimately increase the number of donors recruited (through enhanced information, support, and reassurance during the recruitment process) or it may reduce the financial cost to the recipients of donor sperm, thus making it more affordable to those who are ineligible for state-funded treatment.
The study received no funding from external sources. All authors are Cryos employees or members of the Cryos External Scientific Advisory Committee.
N/A.
Introduction
The successful recruitment of sperm donors is essential to provide a range of medically assisted reproduction (MAR) procedures, which rely upon donor sperm. Typically, these include donor insemination, or IVF using donor sperm by heterosexual couples with severe male factor infertility, and increasingly by single women or women in same-sex relationships (National Institute for Health and Care Excellence (NICE), 2013). Although MAR using donor sperm is highly effective, national legislation typically limits the number of children that can be born from a single donor (Janssens et al., 2011, 2015; Calhaz-Jorge et al., 2020). Therefore, there is a constant requirement for sperm banks to replenish their stocks with sperm from new donors. For example, it has been calculated that at least 400 new donors are required to be recruited each year to meet the need for donor sperm used in MAR in the UK (Hamilton et al., 2008).
While much has been written about the medical screening and assessment of sperm donors from a safety perspective (Clarke et al., 2021; Practice Committee of the American Society for Reproductive Medicine and the Practice Committee for the Society for Assisted Reproductive Technology, 2021), relatively little has been written about the process of recruiting donors and how recruitment processes work in practice. One exception is the paper by Paul et al. (2006) who reported on the outcome of 1101 candidate sperm donors at a single clinic in the northeast of England. They found that only 3.63% of the initial applicants had samples that were frozen and subsequently released for use, with the most common reason for rejection being suboptimal semen quality (85.07%). More recently, Liu et al. (2021) reported on the screening results of 24 040 candidate sperm donors over a 14-year period at a single centre in China and found that 23.38% of candidate donors were accepted. Moreover, they found that acceptance rates were significantly higher for men who were married, with children of their own and who had higher levels of education. They suggested that this was because these men pay more attention to reproductive health and have a lower incidence of sexually transmitted infections.
In a recent study of accepted sperm donors at Cryos in Denmark and the USA (Pennings et al., 2021), some notable differences in demographics and attitudes were found between donors who had chosen to allow their initial identity (ID) to be released to any donor conceived people (ID release) compared to those who had not (non-ID release). For example, ID-release donors were generally older and more likely to have a partner compared to donors who had chosen to be non-ID release. With this in mind, the present study aimed to compare the donor recruitment process in Denmark and the USA and reasoned that the initial ID-release choice of candidate donors may influence the likelihood of them being recruited, either by virtue of the decisions they make as individuals or as a consequence of the biological and demographic differences between them. It also explores whether the outcome of recruitment was influenced by the country in which recruitment took place and at what step the candidate donor journey ended by choice or by selection.
Materials and methods
Data were obtained from the Cryos International (Aarhus, Denmark) databases on every sperm donor applicant in Denmark and the USA within the calendar years 2018 and 2019. The recruitment processes and clinical and laboratory protocols in each country were identical and began when a member of the public filled out an on-line application form available on the Cryos website (https://www.cryosinternational.com). This initial application form included items on basic information (such as age, address) and in 2018 and 2019 their initial preference about whether they wanted to be either an ID-release or non-ID-release donor (see Pennings et al., 2021 for further details), although this decision could be changed later. If the applicant passed this initial check, then candidate donors were invited to provide a semen sample for an assessment of quality and also sperm survival after cryopreservation and thaw. At Cryos, semen samples were cryopreserved in CBS 0.5-ml straws (Cryo Bio System, L’Aigie, France) using either Freezing Medium TYB or Arctic Sperm Cryopreservation Medium (FujiFilm Irvine Scientific, Santa Ana, CA, USA) according to the manufacturer’s instructions. During 2018 and 2019, samples were cooled to −196°C using the method described by Sherman (1973). The minimum semen quality required to be a Cryos donor is for at least 5 × 106 motile sperm/ml to be present in the post-thaw sample. Applicants who did not meet this initial criterion were rejected at this stage and did not receive any financial compensation.
If the post-thaw donor candidate semen quality was adequate, they were then sent a detailed medical questionnaire to complete in their own time and return to Cryos either by e-mail or hard copy. This included items on their lifestyle, medical history, and details about their wider family, including information about their genetic history. In 2018 and 2019, all donors at Cryos were screened in line with the relevant guidelines published by the European Parliament and the Council of the European Union (2004), the Association of Biomedical Andrologists, Association of Clinical Embryologists, British Andrology Society, British Fertility Society, Royal College of Obstetricians & Gynaecologists (2008), and the Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology (2013).
Upon receipt, the completed medical questionnaire was screened by the donor co-ordinators/nurses, in close collaboration with medical doctors and clinical geneticists. If the candidate donor self-declared any high-risk behaviour (e.g. had paid someone for sex or taken recreational drugs intravenously) or a health condition of concern either in themselves or a member of their close family (e.g. a self-diagnosis of psoriasis or family history of a late onset disease such as Huntington’s disease), the donor was rejected at this stage. If anything needed to be clarified or documented further, the candidate donor was asked to provide a copy of his medical records from his family doctor. If the applicant had no obvious risk factors, he was invited to attend a medical consultation, which included an in-person psychological interview with a trained nurse. All applicants that passed the medical screening were then invited to provide a sample of blood and urine, which was used for testing for infectious disease (e.g. HIV, hepatitis B virus (HBV), Chlamydia trachomatis according to the screening guidelines listed above) and for genetic screening. The Cryos approach to genetic screening of sperm donors has been published recently (Payne et al., 2021) and in 2018 and 2019 included testing for 46 recessive disorders. Applicants who passed the genetic and infectious diseases screening and completed the relevant consent forms were invited to start donating. Accepted donors were at liberty to decide in which countries their donations were able to be used by signing country-specific consent forms; thus, donors could limit the number of times they were willing to donate and the number of children that they thought could be acceptably created according to their personal wishes. It was also at this stage that the donor decided whether to agree to provide an extended profile about themselves (see Pennings et al., 2021 for further details).
Typically, as donors at Cryos can donate over several years and they must provide urine and blood samples at regular intervals so that batches of their frozen sperm can be released for use (if tests are negative for HIV, HBV, C. trachomatis, according to the screening guidelines listed above). During 2018 and 2019, in semen samples released for use in the EU, this was done based on nucleic acid amplification testing and serology testing every 90 days, whereas for samples released for use in the USA, this was done every 180 days.
Financial compensation was only made to accepted donors when their samples were certified as being suitable and safe to be released for use (candidate donors who were not accepted, or who withdrew, were not entitled to compensation). The level of compensation was the same in both Denmark and the USA (∼€65–70 per ejaculate depending on the exchange rate). However, donors who agreed to become ID-release donors were entitled to extra financial compensation, as described in Pennings et al. (2021).
Data on the number of donors passing through (or lost) at each stage of the process were provided to the authors for analysis in an anonymized format. Statistical analysis was carried out by Chi squared test, using Graphpad Prism (GraphPad Software, San Diego, CA, USA) to examine differences between location (Denmark or USA) and/or donor type (ID release versus non-ID release) at each stage of the recruitment process. Ethical approval for the secondary analysis of anonymized data was granted by the University of Sheffield Ethics Committee (Ref: 046015).
Results
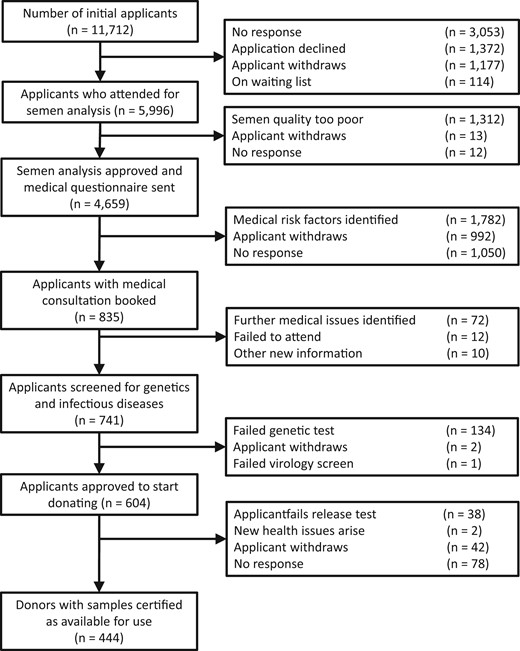
During the calendar years 2018 and 2019, a total of 11 712 men (5878 in Denmark and 5834 in the USA) applied via the Cryos website to be considered a sperm donor (Fig. 1). Of these, 5996 (51.20%) attended for an initial semen analysis and in 4659 men (77.70%), the post-thaw motile sperm concentration was sufficient (>5 × 106 motile sperm/ml) for a copy of the medical questionnaire to be sent to them. Of the applicants who returned the medical questionnaire, a total of 835 were invited for a medical examination and a total of 741 provided sample of blood and urine for genetic and infectious diseases screening. A total of 604 men were found suitable to start donating (5.16% of initial applicants) and a total of 444 (3.79% of initial applicants; 73.51% of those that start donating) had semen samples frozen, analysed after thaw, and passed all screening tests, meaning that their samples were certified as suitable to be released for use.
Overview of the stages of sperm donor recruitment and the stages at which candidate donors are lost or rejected. The stages describe are those carried out by the same organization in both Denmark and the USA.
Taking all these stages into account, Table I shows that, overall, significantly more candidate donors in Denmark were accepted and ultimately had samples certified for used in treatment (6.53%) compared to applicants in the USA (1.03%) (χ2 = 243.2; 1 degree of freedom (df); z = 15.60; P ≤ 0.0001). In addition, statistically more donors who opted at the outset to be ID release went on to be accepted and had samples certified for use (4.70%) compared to those who initially chose to be non-ID release (3.15%) (χ2 = 18.51; 1 df; z = 4.303; P ≤ 0.0001).
Proportion of sperm donors that have samples frozen and certified as safe to use compared to those that initially apply, according to country and initial identity-release preference.
| . | ID release . | Non-ID release . | Total . | . |
|---|---|---|---|---|
| Denmark | 8.91% (197/2210) | 5.10% (187/3668) | 6.53% (384/5878) | χ2 = 243.2; df = 1; z = 15.60; P < 0.0001 |
| USA | 1.14% (30/2623) | 0.93% (30/3211) | 1.03% (60/5834) | |
| Total | 4.70% (227/4833) | 3.15% (217/6879) | 3.79% (444/11 712) | |
| χ2 = 18.51; df = 1; z = 4.303; P < 0.0001 | ||||
| . | ID release . | Non-ID release . | Total . | . |
|---|---|---|---|---|
| Denmark | 8.91% (197/2210) | 5.10% (187/3668) | 6.53% (384/5878) | χ2 = 243.2; df = 1; z = 15.60; P < 0.0001 |
| USA | 1.14% (30/2623) | 0.93% (30/3211) | 1.03% (60/5834) | |
| Total | 4.70% (227/4833) | 3.15% (217/6879) | 3.79% (444/11 712) | |
| χ2 = 18.51; df = 1; z = 4.303; P < 0.0001 | ||||
df: degrees of freedom; ID: identity.
Proportion of sperm donors that have samples frozen and certified as safe to use compared to those that initially apply, according to country and initial identity-release preference.
| . | ID release . | Non-ID release . | Total . | . |
|---|---|---|---|---|
| Denmark | 8.91% (197/2210) | 5.10% (187/3668) | 6.53% (384/5878) | χ2 = 243.2; df = 1; z = 15.60; P < 0.0001 |
| USA | 1.14% (30/2623) | 0.93% (30/3211) | 1.03% (60/5834) | |
| Total | 4.70% (227/4833) | 3.15% (217/6879) | 3.79% (444/11 712) | |
| χ2 = 18.51; df = 1; z = 4.303; P < 0.0001 | ||||
| . | ID release . | Non-ID release . | Total . | . |
|---|---|---|---|---|
| Denmark | 8.91% (197/2210) | 5.10% (187/3668) | 6.53% (384/5878) | χ2 = 243.2; df = 1; z = 15.60; P < 0.0001 |
| USA | 1.14% (30/2623) | 0.93% (30/3211) | 1.03% (60/5834) | |
| Total | 4.70% (227/4833) | 3.15% (217/6879) | 3.79% (444/11 712) | |
| χ2 = 18.51; df = 1; z = 4.303; P < 0.0001 | ||||
df: degrees of freedom; ID: identity.
Table II shows that most candidate donors were lost during the recruitment process because they: withdrew, failed to respond, did not attend an appointment, or failed to return a questionnaire (54.91%); reported a disqualifying health or failed a screening test (17.41%); did not meet the eligibility criteria at the outset (11.71%); or did not have >5 × 106 motile sperm/ml in their post-thaw samples (11.20%). In each case, there were statistically significant differences between countries and the candidate donor’s initial ID choice.
Summary of reasons for candidate donor loss through the recruitment process, by country and initial identity-release choice.
| . | ID release . | Non-ID release . | Total . | . |
|---|---|---|---|---|
| Applicant withdraws or fails to respond (at any stage): | ||||
| Denmark | 42.35% (936/2210) | 52.48% (1925/3668) | 48.67% (2861/5878) | χ2 = 185.4; df = 1; z = 13.62; P < 0.0001 |
| USA | 59.59% (1563/2623) | 62.50% (2007/3211) | 61.19% (3570/5834) | |
| Total | 51.71% (2499/4833) | 57.16% (3932/6879) | 54.91% (6431/11 712) | |
| χ2 = 34.08; df = 1; z = 5.838; P < 0.0001 | ||||
| Rejected owing to a health issue or because of failure of a genetic test or one or more tests for infectious diseases (at any stage): | ||||
| Denmark | 15.66% (346/2210) | 12.73% (467/3668) | 14.29% (813/5878) | χ2 = 105.1; df = 1; z = 10.25; P < 0.0001 |
| USA | 22.15% (581/2623) | 20.09% (645/3211) | 21.01% (1226/5834) | |
| Total | 19.18% (927/4833) | 16.17% (1112/6879) | 17.41% (2039/11 712) | |
| χ2 = 17.95; df = 1; z = 4.237; P < 0.0001 | ||||
| Rejected at initial screening questionnaire: | ||||
| Denmark | 15.43% (341/2210) | 11.64% (427/3668) | 13.37% (768/5878) | χ2 = 20.83; df = 1; z = 4.564; P < 0.0001 |
| USA | 9.91% (260/2623) | 10.71% (344/3211) | 10.35% (604/5834) | |
| Total | 12.44% (601/4833) | 11.21% (771/6879) | 11.71% (1372/11 712) | |
| χ2 = 4.134; df = 1; z = 2.033; P = 0.0420 | ||||
| Rejected because of poor semen quality: | ||||
| Denmark | 17.51% (387/2210) | 15.08% (553/3668) | 15.99% (940/5878) | χ2 = 272.1; df = 1; z = 16.50; P < 0.0001 |
| USA | 7.17% (188/2623) | 5.73% (184/3211) | 6.38% (372/5834) | |
| Total | 11.90% (575/4833) | 10.71% (737/6879) | 11.20% (1312/11 712) | |
| χ2 = 3.998; df = 1; z = 1.999; P = 0.0456 | ||||
| . | ID release . | Non-ID release . | Total . | . |
|---|---|---|---|---|
| Applicant withdraws or fails to respond (at any stage): | ||||
| Denmark | 42.35% (936/2210) | 52.48% (1925/3668) | 48.67% (2861/5878) | χ2 = 185.4; df = 1; z = 13.62; P < 0.0001 |
| USA | 59.59% (1563/2623) | 62.50% (2007/3211) | 61.19% (3570/5834) | |
| Total | 51.71% (2499/4833) | 57.16% (3932/6879) | 54.91% (6431/11 712) | |
| χ2 = 34.08; df = 1; z = 5.838; P < 0.0001 | ||||
| Rejected owing to a health issue or because of failure of a genetic test or one or more tests for infectious diseases (at any stage): | ||||
| Denmark | 15.66% (346/2210) | 12.73% (467/3668) | 14.29% (813/5878) | χ2 = 105.1; df = 1; z = 10.25; P < 0.0001 |
| USA | 22.15% (581/2623) | 20.09% (645/3211) | 21.01% (1226/5834) | |
| Total | 19.18% (927/4833) | 16.17% (1112/6879) | 17.41% (2039/11 712) | |
| χ2 = 17.95; df = 1; z = 4.237; P < 0.0001 | ||||
| Rejected at initial screening questionnaire: | ||||
| Denmark | 15.43% (341/2210) | 11.64% (427/3668) | 13.37% (768/5878) | χ2 = 20.83; df = 1; z = 4.564; P < 0.0001 |
| USA | 9.91% (260/2623) | 10.71% (344/3211) | 10.35% (604/5834) | |
| Total | 12.44% (601/4833) | 11.21% (771/6879) | 11.71% (1372/11 712) | |
| χ2 = 4.134; df = 1; z = 2.033; P = 0.0420 | ||||
| Rejected because of poor semen quality: | ||||
| Denmark | 17.51% (387/2210) | 15.08% (553/3668) | 15.99% (940/5878) | χ2 = 272.1; df = 1; z = 16.50; P < 0.0001 |
| USA | 7.17% (188/2623) | 5.73% (184/3211) | 6.38% (372/5834) | |
| Total | 11.90% (575/4833) | 10.71% (737/6879) | 11.20% (1312/11 712) | |
| χ2 = 3.998; df = 1; z = 1.999; P = 0.0456 | ||||
NB: Excluded from this table are 114 candidate donors (112 in Denmark and 2 in the USA) who were placed on a waiting list during the recruitment process and whose recruitment was therefore paused because they did not proceed to semen analysis (see Fig. 1).
df: degrees of freedom; ID: identity.
Summary of reasons for candidate donor loss through the recruitment process, by country and initial identity-release choice.
| . | ID release . | Non-ID release . | Total . | . |
|---|---|---|---|---|
| Applicant withdraws or fails to respond (at any stage): | ||||
| Denmark | 42.35% (936/2210) | 52.48% (1925/3668) | 48.67% (2861/5878) | χ2 = 185.4; df = 1; z = 13.62; P < 0.0001 |
| USA | 59.59% (1563/2623) | 62.50% (2007/3211) | 61.19% (3570/5834) | |
| Total | 51.71% (2499/4833) | 57.16% (3932/6879) | 54.91% (6431/11 712) | |
| χ2 = 34.08; df = 1; z = 5.838; P < 0.0001 | ||||
| Rejected owing to a health issue or because of failure of a genetic test or one or more tests for infectious diseases (at any stage): | ||||
| Denmark | 15.66% (346/2210) | 12.73% (467/3668) | 14.29% (813/5878) | χ2 = 105.1; df = 1; z = 10.25; P < 0.0001 |
| USA | 22.15% (581/2623) | 20.09% (645/3211) | 21.01% (1226/5834) | |
| Total | 19.18% (927/4833) | 16.17% (1112/6879) | 17.41% (2039/11 712) | |
| χ2 = 17.95; df = 1; z = 4.237; P < 0.0001 | ||||
| Rejected at initial screening questionnaire: | ||||
| Denmark | 15.43% (341/2210) | 11.64% (427/3668) | 13.37% (768/5878) | χ2 = 20.83; df = 1; z = 4.564; P < 0.0001 |
| USA | 9.91% (260/2623) | 10.71% (344/3211) | 10.35% (604/5834) | |
| Total | 12.44% (601/4833) | 11.21% (771/6879) | 11.71% (1372/11 712) | |
| χ2 = 4.134; df = 1; z = 2.033; P = 0.0420 | ||||
| Rejected because of poor semen quality: | ||||
| Denmark | 17.51% (387/2210) | 15.08% (553/3668) | 15.99% (940/5878) | χ2 = 272.1; df = 1; z = 16.50; P < 0.0001 |
| USA | 7.17% (188/2623) | 5.73% (184/3211) | 6.38% (372/5834) | |
| Total | 11.90% (575/4833) | 10.71% (737/6879) | 11.20% (1312/11 712) | |
| χ2 = 3.998; df = 1; z = 1.999; P = 0.0456 | ||||
| . | ID release . | Non-ID release . | Total . | . |
|---|---|---|---|---|
| Applicant withdraws or fails to respond (at any stage): | ||||
| Denmark | 42.35% (936/2210) | 52.48% (1925/3668) | 48.67% (2861/5878) | χ2 = 185.4; df = 1; z = 13.62; P < 0.0001 |
| USA | 59.59% (1563/2623) | 62.50% (2007/3211) | 61.19% (3570/5834) | |
| Total | 51.71% (2499/4833) | 57.16% (3932/6879) | 54.91% (6431/11 712) | |
| χ2 = 34.08; df = 1; z = 5.838; P < 0.0001 | ||||
| Rejected owing to a health issue or because of failure of a genetic test or one or more tests for infectious diseases (at any stage): | ||||
| Denmark | 15.66% (346/2210) | 12.73% (467/3668) | 14.29% (813/5878) | χ2 = 105.1; df = 1; z = 10.25; P < 0.0001 |
| USA | 22.15% (581/2623) | 20.09% (645/3211) | 21.01% (1226/5834) | |
| Total | 19.18% (927/4833) | 16.17% (1112/6879) | 17.41% (2039/11 712) | |
| χ2 = 17.95; df = 1; z = 4.237; P < 0.0001 | ||||
| Rejected at initial screening questionnaire: | ||||
| Denmark | 15.43% (341/2210) | 11.64% (427/3668) | 13.37% (768/5878) | χ2 = 20.83; df = 1; z = 4.564; P < 0.0001 |
| USA | 9.91% (260/2623) | 10.71% (344/3211) | 10.35% (604/5834) | |
| Total | 12.44% (601/4833) | 11.21% (771/6879) | 11.71% (1372/11 712) | |
| χ2 = 4.134; df = 1; z = 2.033; P = 0.0420 | ||||
| Rejected because of poor semen quality: | ||||
| Denmark | 17.51% (387/2210) | 15.08% (553/3668) | 15.99% (940/5878) | χ2 = 272.1; df = 1; z = 16.50; P < 0.0001 |
| USA | 7.17% (188/2623) | 5.73% (184/3211) | 6.38% (372/5834) | |
| Total | 11.90% (575/4833) | 10.71% (737/6879) | 11.20% (1312/11 712) | |
| χ2 = 3.998; df = 1; z = 1.999; P = 0.0456 | ||||
NB: Excluded from this table are 114 candidate donors (112 in Denmark and 2 in the USA) who were placed on a waiting list during the recruitment process and whose recruitment was therefore paused because they did not proceed to semen analysis (see Fig. 1).
df: degrees of freedom; ID: identity.
Finally, during the screening process, some candidate donors decided to change ID type before their final consent forms were signed and their samples certified for use (Table III). Overall, there was no difference between Denmark and the USA in the frequency in which this occurred (χ2 = 0.2852; 1 df; z = 0.5340; P = 0.5933), but this decision did differ between their initial ID-release preference: it was more common for a donor to change from non-ID release to ID release (27.19%) than the other way around (11.45%) (χ2 = 17.75; 1 df; z = 4.213; P < 0.0001), although movements in both directions did occur in both countries.
Proportion of donors that have samples frozen and certified as safe to use that decided to swap from non-identity release to identity release (or vice versa) during the donation process.
| . | ID release to non-ID release . | Non-ID release to ID release . | Total . | . |
|---|---|---|---|---|
| Denmark | 11.68% (23/197) | 26.20% (49/187) | 18.75% (72/384) | χ2 = 0.2852; df = 1; z = 0.5340; P = 0.5933 |
| USA | 10.00% (3/30) | 33.33% (10/30) | 21.67% (13/60) | |
| Total | 11.45% (26/227) | 27.19% (59/217) | 19.14% (85/444) | |
| χ2 = 17.75; df = 1; z = 4.213; P < 0.0001 | ||||
| . | ID release to non-ID release . | Non-ID release to ID release . | Total . | . |
|---|---|---|---|---|
| Denmark | 11.68% (23/197) | 26.20% (49/187) | 18.75% (72/384) | χ2 = 0.2852; df = 1; z = 0.5340; P = 0.5933 |
| USA | 10.00% (3/30) | 33.33% (10/30) | 21.67% (13/60) | |
| Total | 11.45% (26/227) | 27.19% (59/217) | 19.14% (85/444) | |
| χ2 = 17.75; df = 1; z = 4.213; P < 0.0001 | ||||
df: degrees of freedom; ID: identity.
Proportion of donors that have samples frozen and certified as safe to use that decided to swap from non-identity release to identity release (or vice versa) during the donation process.
| . | ID release to non-ID release . | Non-ID release to ID release . | Total . | . |
|---|---|---|---|---|
| Denmark | 11.68% (23/197) | 26.20% (49/187) | 18.75% (72/384) | χ2 = 0.2852; df = 1; z = 0.5340; P = 0.5933 |
| USA | 10.00% (3/30) | 33.33% (10/30) | 21.67% (13/60) | |
| Total | 11.45% (26/227) | 27.19% (59/217) | 19.14% (85/444) | |
| χ2 = 17.75; df = 1; z = 4.213; P < 0.0001 | ||||
| . | ID release to non-ID release . | Non-ID release to ID release . | Total . | . |
|---|---|---|---|---|
| Denmark | 11.68% (23/197) | 26.20% (49/187) | 18.75% (72/384) | χ2 = 0.2852; df = 1; z = 0.5340; P = 0.5933 |
| USA | 10.00% (3/30) | 33.33% (10/30) | 21.67% (13/60) | |
| Total | 11.45% (26/227) | 27.19% (59/217) | 19.14% (85/444) | |
| χ2 = 17.75; df = 1; z = 4.213; P < 0.0001 | ||||
df: degrees of freedom; ID: identity.
Discussion
This analysis shows that, during 2018 and 2019, only a small fraction of initial applicants at Cryos in Denmark and the USA were finally accepted as sperm donors and passed all the screening tests such that their frozen samples were certified as being suitable and safe to be released for use. This figure (3.79%) is very similar to that reported by Paul et al. (2006) in the Northeast of England (3.63%), but significantly lower that recently reported by Liu et al. (2021) in China (23.38%). However, in their numerator, Liu et al. (2021) only reported applicants who had previously met the basic requirements, and this probably corresponds closer to the 5996 applicants in our study who passed the initial screening questionnaire (Fig. 1) and who then attended for semen analysis. When this figure is used as the numerator instead, our final acceptance rate increases slightly to 7.40%, which is still remarkably low in comparison. The recruitment rates for other countries include: 14.8% in Nigeria (Akinrinola et al., 2003), 32% in the USA (Schroeder-Jenkins and Rothmann 1989), and 33.2% in China (Ping et al., 2011). Liu et al. (2021) suggested that these country differences in the final acceptance rate may be caused by different recruitment criteria in operation at each location. However, in this study, we have been able to show that there were genuine country differences between Denmark and the USA in the overall acceptance rate, given that Cryos uses the same policies, procedures, and protocols in both countries. Briefly, candidate donor applicants in Denmark were over 6.3 times more likely to be accepted and have samples certified as suitable for release and use in treatment in comparison to applicants in the USA (Table I). This was for a variety of different reasons.
First, it was more common in the USA than in Denmark (61.19% versus 48.67%) for candidate donors to be lost from recruitment because they withdrew or did not respond (Table II). However, the stage at which this happened was very different: 94.65% of candidate donor withdrawals in Denmark occur immediately after the application was submitted but before they ever attended for semen analysis and test cryopreservation, whereas in the USA, 55.52% of withdrawals occur after the candidate donors had passed this stage and had been sent the medical questionnaire to complete. This marked country difference could suggest that many of the initial applicants in Denmark may not be serious at the outset or are afraid of being rejected and therefore quickly withdraw, but this is harder to explain for candidate donor applicants in the USA. It could be that in the USA, candidate donors who pass the semen assessment are then put off by the information they are required to disclose in the medical questionnaire once they see it. This may be because they have concerns about how this might impact on their health insurance, or because they are aware of lifestyle behaviours they have that they would rather not disclose. Alternatively, it could be that men in the USA apply to be candidate donors only to obtain access to a free semen analysis (given the relatively poor coverage of male factor infertility by health insurance—see Glazer et al., 2020) and once this is done, they have no interest in progressing any further as a donor. Either way, a further study is required to understand this difference in greater detail.
Second, across the whole recruitment pathway, more candidate donors in the USA (21.01% versus 14.29% in Denmark) were rejected because of a health issue, detected by questionnaire, a genetic test, or a screen for infectious diseases (Table II). This suggests that candidate donors in Denmark and the USA are not identical in their health status, or their willingness to disclose their medical history. It is also possible that this is influenced by the fact that guidelines in the USA (Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology, 2013) are arguably more stringent than the comparable guidelines in Denmark (European Parliament and the Council of the European Union, 2004; the Association of Biomedical Andrologists, Association of Clinical Embryologists, British Andrology Society, British Fertility Society, Royal College of Obstetricians & Gynaecologists, 2008). For example, candidate donors in the USA who have lived in Europe cannot be accepted as donors owing to the perceived increased ‘risk’ of Creutzfeldt–Jakob disease. However, we do not consider that this issue can wholly explain the higher rejection of donors because of health issues in the USA.
Third, more candidate donors in Denmark (13.37%) were rejected at the initial screening questionnaire stage compared to in the USA (10.35%), although the difference was relatively small (Table II). Finally, candidate donors in Denmark were 2.5 times more likely to be rejected because their semen samples failed to achieve the required >5 × 106 motile sperm/ml in their post-thaw sample compared to those in the USA (Table II). This may reflect country-level differences in their semen quality, given that laboratory protocols were the same in both countries, but could also be a consequence of country differences in response to cryopreservation. In previous studies, poor semen quality was a major reason for candidate donor rejection (from 90.27% in Liu et al., 2021 to 55.0% in Ping et al., 2011), which are much higher values than the average 11.20% described in this study (Table II).
In addition to country-level differences, applicants who at the outset chose to be ID release were also significantly more likely to be accepted and have their samples frozen and certified as safe and available for treatment (4.70%) compared to those who initially chose non-ID release (3.15%), although the difference was quite small. Again, in both groups, the main reason for donors to be lost was because they withdrew or did not respond (Table II), but interestingly this was less common in those who chose to be ID release (51.71%) compared to those who did not (57.16%). This may suggest that those who chose ID release have a greater motivation to be a donor at the outset and once they have applied are less likely to walk away. Interestingly, statistically more candidate donors who chose ID release (19.18%) were rejected because they reported a disqualifying health issue or failed a screening test (Table II) compared to those who chose non-ID release (16.17%). Although a relatively small difference, this may be explained by the fact that ID-release donors are generally older (Pennings et al., 2021) and so would have more opportunity for a disqualifying health issue to appear (Gunderson et al., 2022). Regarding those donors who were rejected at the initial screening questionnaire stage (Table II) and rejected because of their post-thaw semen quality (Table II), in both cases, it was statistically more likely that ID-release donors would be, although the differences were again quite small. We can think of no a priori reason why the initial semen quality and post-thaw motile concentration should differ because of a candidate donors ID choice, but there may exist demographic differences between the groups about which we did not have any information (see below).
An interesting observation in the dataset is the frequency with which candidate donors changed their ID choice selected when they first applied, compared to the decision they made when the final consent forms were signed (Table III). It is noteworthy that more of the accepted and released donors who began by selecting non-ID release opt to change to ID release (27.19%) than the other way around (11.45%); the former may be explained by the counselling and general familiarity that will happen as the candidate donor starts donating and gets used to the recruitment process. However, we cannot discount that it is primarily driven by the financial reward that is given to ID-release donors (Cohen et al., 2016). We have described previously that donors who are willing to be identifiable at Cryos are entitled to extra financial compensation (Pennings et al., 2021). However, this cannot explain why candidate donors might initially make the decision to be ID release but then subsequently change their mind. Such ‘cold feet’ requires further exploration and would be an interesting question for a future study.
There are many strengths to this study, including the sheer scale of the numbers of candidate donors involved and the fact that we were able to examine donor recruitment simultaneously in two countries occurring within the same organization and using the same protocols. While we cannot completely discount some local variation in record keeping or decision-making from time to time, we think that this is minimized and does not represent a systematic issue. However, a limitation of this study is that we only had anonymized summary data to work with and we did not have any information about basic demographic characteristics of the applicants (e.g. ethnicity and age) or any information about their lifestyle or medical history that could have accounted for some of the country and ID-release differences seen in donor recruitment and which may have influenced their chances of being accepted as a donor.
In conclusion, this study provides further evidence to illustrate how challenging sperm donor recruitment processes are, with only a small fraction of those who initially apply ultimately being accepted and having samples certified as safe for use in treatment. It highlights how this can be influenced by the country in which recruitment takes place as well as the ID-release decisions made by the candidate donor at the outset. Given the investment of time and resources into the various stages of sperm donor recruitment, we suggest that efficiencies may be possible by developing country-specific donor recruitment pathways, which take account of some of the issues described here. It may also be worthwhile to conduct further research to better understand when and why so many candidate donors are lost in the recruitment process. This could ultimately increase the number of donors recruited (through enhanced information, support, and reassurance during the recruitment processes), while maintaining the high standards of selection for safety and clinical effectiveness of the donations, which are eventually released for use. In addition, more efficient systems may reduce the financial cost of donor recruitment and therefore lower the price of donor sperm, thus making it more affordable to those who are ineligible for state-funded treatment.
Data availability
The data underlying this article were provided by Cryos International (Aarhus, Denmark) by permission. Data will be shared on request to the corresponding author with the permission of Cryos International.
Acknowledgements
The authors would like to thank Jeanett Bukdahl and Tobias Dahl at Cryos for help in collating the data for analysis.
Authors’ roles
The study was designed by A.A.P., G.P., and A.-B.S. A.A.P. undertook the data analysis and drafted the paper. All authors contributed to the writing of the article and approved the final version.
Funding
The study received no funding.
Conflict of interest
C.B. and A.-B.S. are Cryos employees. A.A.P., G.P., E.M., J.R., A.P., and S.W.A. are all members of the Cryos External Scientific Advisory Committee (ESAC).