-
PDF
- Split View
-
Views
-
Cite
Cite
Yu Liu, Tao Lu, Yongxing Zhang, Yulei Qiao, Junjie Xi, Qun Wang, Collagen-conjugated tracheal prosthesis tested in dogs without omental wrapping and silicone stenting, Interactive CardioVascular and Thoracic Surgery, Volume 23, Issue 5, November 2016, Pages 710–715, https://doi.org/10.1093/icvts/ivw240
Close - Share Icon Share
Abstract
Artificial tracheas fabricated from collagen-conjugated mesh appear to overcome fatal postoperative complications, namely anastomotic dehiscence and prosthesis dislocation. Such prostheses are incorporated by host tissue, provided they are wrapped in omentum (necessitating an additional abdominal procedure) and a silicone tube is used as a stent (to be extracted several weeks postoperatively). To mitigate related host impact (i.e. injury, pain and distress), we investigated the feasibility of implanting this type of tracheal prosthesis (∼5 cm in length) alone, without omental wrapping and use of a silicone stent.
Porous-type tracheal prostheses that were reinforced with a continuous polypropylene spiral and sealed by collagen sponge from porcine skin replaced segments of cervical trachea (∼5 cm long) in 10 dogs through the method of telescopic anastomosis. Omental wrapping and silicone stent placement were omitted. Postoperatively, bronchoscopic examination was performed periodically. When dogs died or were sacrificed, tracheal prostheses were harvested for haematoxylin and eosin staining and electron microscopic scanning of luminal surface conditions.
With the exception of one death from an anaesthesia-related incident during fibre-optic bronchoscopy (postsurgical week 1), nine dogs survived uneventfully (until sacrifice), without prosthesis dislocation or anastomotic dehiscence. The longest observation period was 2 years and 8 months. Bronchoscopic examination revealed that no stenosis or local infection was evident in the prostheses of five dogs. Moderate (n = 2) and slight (n = 2) stenoses were observed in the other four animals. All four animals survived for a long time, without dyspnoea or stridor. Histological examination showed that partial inner surface of the artificial trachea was covered with the pseudostratified ciliated epithelium. Regeneration of ciliated epithelium was also confirmed by scanning electron microscopy.
This pilot study revealed that implantation of a collagen-conjugated tracheal prosthesis (∼5 cm in length) is feasible without the need for omental wrapping and silicone stenting as ancillary measures. This approach considerably simplified the surgical procedures to minimize host intrusion, which indicated a possible clinical application.
INTRODUCTION
In patients with tracheal malignancies or tracheal stenosis from benign causes, the anastomotic tension produced by lengthy (>6 cm) tracheal resections predisposes to anastomotic dehiscence—a fatal postoperative complication [1]. Currently, thoracic surgeons do not perform radical tracheal resections. Although rebuilding the airway via an artificial trachea is a solution that many researchers have rigorously pursued, most prostheses are plagued by poor biocompatibility [2], making the healing process difficult. Anastomotic dehiscence, prosthesis dislocation or luminal stenosis may lead to implant failure. Some situations may result in deadly bleeding from the innominate artery. After countless experiments, a prototypic prosthesis, based on collagen conjugate technology, seems to have overcome such complications by virtue of its high biocompatibility. This prosthesis is better incorporated by host tissue [3–6] and has been applied for the repair of the partial tracheal defect in humans successfully [7–9].
In the adult trachea, which is ∼12 cm in length, segmental resection up to 6 cm is feasible. Most surgical needs are adequately met by an artificial trachea measuring 5 cm. At this length, a collagen-conjugated artificial trachea requires omental wrapping and a silicone tube as a stent. The omental wrap promotes revascularization and epithelial regeneration, and the silicone tube is placed to ensure clearance of sputum and to prevent infection [4–6]. Still, these measures have drawbacks. An added abdominal incision is needed to prepare the vascularized omentum, and the omental pedicle graft must be delivered to the anastomotic site through the diaphragm. The host thus endures greater trauma. Moreover, asymptomatic diaphragmatic hernias have been found in related studies of ours [10–12] involving animals sacrificed after omental wrapping, and endobronchial silicon tube extraction (4–8 weeks after surgery) is yet another procedure the host must endure. Early collagen-conjugated tracheal prostheses, used without omental wrapping and silicone stenting in animal experiments, generated only 2 cm lengths [3] that were inadequate for clinical needs. There are no data on tracheal replacement by 5-cm-long collagen-conjugated prostheses implanted without omental wrapping and silicone tube insertion.
To reduce the cumulative impact on the host (i.e. injury, pain and distress) imposed by this procedure, we studied a prosthesis devised for implantation in 5 cm lengths, without the need of omental wrapping and silicone stenting. Surgical procedures were thus considerably simplified. Preliminary results of animal implantation are presented herein.
MATERIALS AND METHODS
Extraction of collagen
Collagen was extracted from porcine skin through a process similar to that of Okumura and coworkers [3]. In brief, the porcine skin was first finely chopped into very small pieces. After immersing the mince in cold hydrochloric acid solution (HCl, 0.01 mol/l, Sigma-Aldrich Corp, Shanghai, China) for 24 h, it was homogenized with Polytron material (Kinematika, Lucerne, Switzerland). Following a 48-h treatment with pepsin (2%, Sigma-Aldrich Corp) at 4°C, the homogenate was centrifuged at 7000 rpm for 20 min. Ultimately, the pellet was discarded, leaving a supernatant of Type I (70–80%) and Type III (20–30%) collagen, which was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) [3]. The telopeptide of collagen, which was responsible for the antigenicity, was essentially devoid.
Preparation of tracheal prosthesis
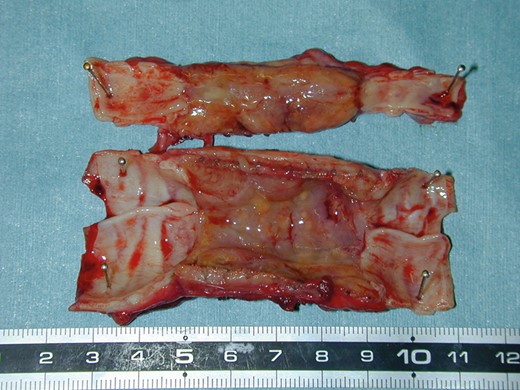
Preparation of our tracheal prosthesis relied on the technique of Teramachi et al. [5]. To summarize, a cylinder (diameter, 2 cm; length, 6 cm) was fashioned from fine Marlex mesh (pore size, 260 μm, C.R. Bard, Inc., Billerica, MA, USA) reinforced at the exterior by a spiral polypropylene line (1 mm diameter) at 5 mm intervals (Fig. 1, top). The mesh cylinder was first exposed to a plasma discharge to introduce peroxides onto its surface, with the aim of ensuring that graft polymerization of acrylic acid on the cylinder surface could be performed. Then collagen extracted from porcine skin was immobilized on its surface by graft polymerization of acrylic acid. The cylinder was then coated thickly with 1.3% collagen to prevent air leakage from the pores of the mesh. Subsequently, with the purpose of making the collagen cross-linked on the surface of the artificial trachea, a 15 W ultraviolet lamp was used as a light source to irradiate the prosthesis constantly for 16 h [3]. Once coated inside and outside, the prosthesis was immersed in a mold filled with the same 1.3% collagen. To fortify the collagenous shell, the mould was freeze-dried and then heated up at 105°C in a vacuum for 12 h (Fig. 1, bottom).
Preparation of the tracheal prosthesis: A cylinder (diameter, 2 cm; length, 6 cm) is fabricated from fine Marlex mesh and reinforced at its exterior by a spiral polypropylene line (top). First coated with porcine collagen (inside/outside), the prosthesis is then immersed in a collagen-filled mould, freeze-dried and heated in vacuum (bottom).
Implantation
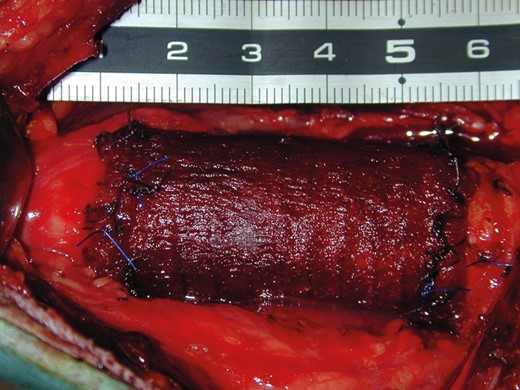
Ten adult mongrel dogs (weight, 8–17 kg) were selected for testing. After endotracheal intubation (size, 7.0 mm inner diameter, 9.6 mm outer diameter) of the dog, anaesthesia was maintained with 50% oxygen, 50% nitrous oxide and 1% halothane. Exposure of the cervical trachea was prepared by a midline incision in the neck. After a 5 cm segment of the cervical trachea was resected, a sterile flexible intubation tube was inserted into the distal cut end of the trachea to maintain ventilation and anaesthesia on the operating table. Then the defect was reconstructed with the prepared prosthesis. Telescopic anastomosis was performed by interrupted suturing with 3-0 Vicryl sutures (Ethicon, Inc., Somerville, NJ, USA) as follows. The proximal cut end of the host trachea was sutured to the prosthesis first, and then anastomosis of the distal cut end was performed in the same manner. After the procedures, the intubation tube that was inserted into the trachea on the operating table was removed and respiration was transferred to transoral intubation to maintain ventilation and anaesthesia. Integrity of the respiratory tract was thereby re-established (Fig. 2), without omental wrapping or use of a silicone tube. The surgery lasted an hour.
Implantation of the tracheal prosthesis: Cervical trachea (∼5 cm in length) replaced by artificial trachea; no omental wrapping or use of a silicone stent.
Postoperative treatment and observation
After induction of general anaesthesia by an intramuscular injection of ketamine hydrochloride (10 mg/kg) and xylazine hydrochloride (4 mg/kg), bronchoscopic examination was performed periodically once a week in the first month. Subsequently, this examination was conducted repeatedly once a month until the dog was sacrificed. The luminal surface was observed by flexible bronchoscopy to check for respiratory tract stenosis and hyperproliferative granulation tissue. Stenosis was gauged as follows: slight stenosis, less than one-third reduction in the lumen of the prosthesis and luminal surface irregularity; moderate stenosis, more than one-third but less than two-thirds reduction in the lumen of the prosthesis; and severe stenosis, more than two-thirds reduction in the lumen of the prosthesis. When animals died or were sacrificed, the artificial tracheas were harvested for macroscopic evaluation and then the specimens of the prostheses were prepared for haematoxylin and eosin (HE) staining and scanning electron microscopy (SEM) of luminal surface conditions.
A dual antibiotic regimen (ampicillin sodium and cloxacillin sodium, 250 mg/day each) was given intramuscularly during postoperative week 1 and then orally until the end of week 4.
All animals were treated humanely, in compliance with the eighth edition of the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Institutes of Health (NIH, revised 2011).
RESULTS
At postoperative week 1, one dog died of an anaesthesia-related incident during fibre-optic bronchoscopy. All nine dogs that remained survived uneventfully (until sacrifice), without prosthesis dislocation or anastomotic dehiscence. No animals died from complications related to the prosthesis. The longest observation period was 2 years and 8 months (Table 1).
Outcomes of implanted tracheal prostheses
| No. . | Observation time . | Anastomotic dehiscence . | Prosthesis dislocation . | Stenosis . | Local infection . |
|---|---|---|---|---|---|
| 1 | 1 week | No | No | No | No |
| 2 | 2 months | No | No | No | No |
| 3 | 3 months | No | No | Slight | No |
| 4 | 4 months | No | No | No | No |
| 5 | 5 months | No | No | Moderate | No |
| 6 | 10 months | No | No | Moderate | No |
| 7 | 10 months | No | No | No | No |
| 8 | 13 months | No | No | Slight | No |
| 9 | 18 months | No | No | No | No |
| 10 | 32 months | No | No | No | No |
| No. . | Observation time . | Anastomotic dehiscence . | Prosthesis dislocation . | Stenosis . | Local infection . |
|---|---|---|---|---|---|
| 1 | 1 week | No | No | No | No |
| 2 | 2 months | No | No | No | No |
| 3 | 3 months | No | No | Slight | No |
| 4 | 4 months | No | No | No | No |
| 5 | 5 months | No | No | Moderate | No |
| 6 | 10 months | No | No | Moderate | No |
| 7 | 10 months | No | No | No | No |
| 8 | 13 months | No | No | Slight | No |
| 9 | 18 months | No | No | No | No |
| 10 | 32 months | No | No | No | No |
Outcomes of implanted tracheal prostheses
| No. . | Observation time . | Anastomotic dehiscence . | Prosthesis dislocation . | Stenosis . | Local infection . |
|---|---|---|---|---|---|
| 1 | 1 week | No | No | No | No |
| 2 | 2 months | No | No | No | No |
| 3 | 3 months | No | No | Slight | No |
| 4 | 4 months | No | No | No | No |
| 5 | 5 months | No | No | Moderate | No |
| 6 | 10 months | No | No | Moderate | No |
| 7 | 10 months | No | No | No | No |
| 8 | 13 months | No | No | Slight | No |
| 9 | 18 months | No | No | No | No |
| 10 | 32 months | No | No | No | No |
| No. . | Observation time . | Anastomotic dehiscence . | Prosthesis dislocation . | Stenosis . | Local infection . |
|---|---|---|---|---|---|
| 1 | 1 week | No | No | No | No |
| 2 | 2 months | No | No | No | No |
| 3 | 3 months | No | No | Slight | No |
| 4 | 4 months | No | No | No | No |
| 5 | 5 months | No | No | Moderate | No |
| 6 | 10 months | No | No | Moderate | No |
| 7 | 10 months | No | No | No | No |
| 8 | 13 months | No | No | Slight | No |
| 9 | 18 months | No | No | No | No |
| 10 | 32 months | No | No | No | No |
Bronchoscopic and macroscopic examination
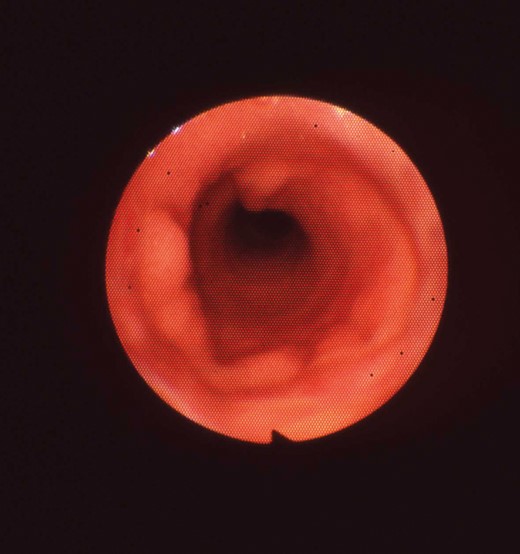
In five dogs, bronchoscopic examination revealed that neither stenosis nor granulation was evident at the anastomosis sites and the luminal surfaces of the prostheses in the whole observation period (Fig. 3). No mesh exposure was observed in the lumen of the prostheses and the generated mucosa appeared lustrous and reddish. One of these dogs was sacrificed at 2 months, one at 4 months, one at 10 months, one at 18 months and one at 32 months after implantation. When removing the implants after sacrificing the animals, the prostheses were observed to be incorporated into the host trachea and adhere to the adjacent tissues. The luminal surfaces of the prostheses were patent, shiny and devoid of retained sputum or local infection at the macroscopic level (Fig. 4).
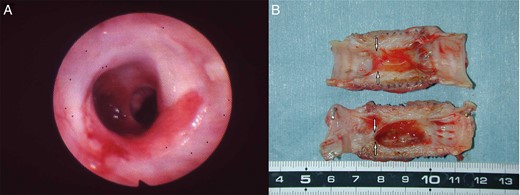
By bronchoscopy, no luminal stenosis or sputum retention post implantation (month 32) of the prosthesis in dog 10.
Luminal surface of the prosthesis post implantation (month 32) in dog 10 appears shiny and patent at the macroscopic level, with no evidence of local infection or anastomotic dehiscence.
Moderate stenosis was found at the proximal end of the prostheses in two dogs, but dislocation and anastomotic dehiscence were not seen (Fig. 5A and B). One of the two dogs was observed for 5 months, and retention of the sputum or local infection was not seen inside the prosthesis. The other one was observed for a 10-month period, during which the observed stenosis did not progress. The prostheses of two other dogs also displayed slight stenosis and were observed for 3 months and 13 months, respectively. All four animals survived until they were sacrificed on schedule, without dyspnoea or stridor.
(A) By bronchoscopy, moderate stenosis was seen post implantation (month 10) at the proximal end of the prosthesis in dog 6. (B) No anastomotic dehiscence on a macroscopic post-implantation view of the prosthesis but moderate stenosis was present (arrow).
Histological examination
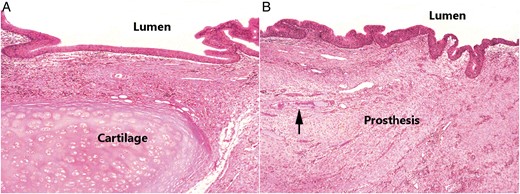
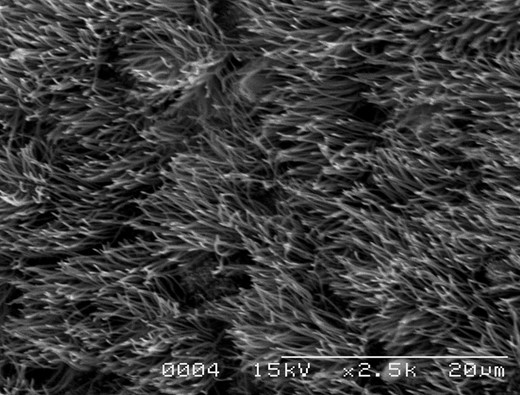
Histological examination of HE staining revealed that the inner surfaces of the prostheses were covered with varying degree of regenerated epithelium. Moreover, pseudostratified ciliated columnar epithelium was recognized on partial inner surfaces. Regenerated epithelium extended from the anastomotic sites to the prostheses. The host tracheal tissue near the anastomotic sites was composed of mucosa (pseudostratified ciliated columnar epithelium and lamina propria), submucosa (including loose connective tissue) and cartilage (Fig. 6A). Correspondingly, the surface of the artificial trachea near the anastomotic sites was covered with regenerated pseudostratified ciliated columnar epithelium. Adjacent connective tissue (including small vessels) had invaded through the pores of the mesh, and the prostheses were incorporated by host tissue (Fig. 6B). SEM revealed luminal ingrowth of regenerated epithelium that possessed cilia similar to that of the normal tracheal epithelium (Fig. 7).
(A) Light microscopic view of normal histological structure of the host trachea near the anastomotic site, including mucosa, submucosa and cartilage. (B) Neomucosa with epithelial lining on the mesh near the anastomotic site. The surface of the prosthesis was covered with ciliated columnar epithelium. Adjacent connective tissue had invaded through the pores of the mesh with ingrowth of some vessels (arrow). (Both, haematoxylin and eosin staining; original magnification, ×40).
Scanning electron microscopy showing partial layering of prosthetic luminal surface by ciliated epithelium (×1500).
DISCUSSION
In the past, various porous synthetic materials were applied for tracheal reconstruction. Pearson and coworkers [13] initiated laboratory studies with heavy Marlex mesh (polyethylene) and subsequently applied it clinically to replace the trachea in 2 patients. They obtained some degree of success. However, the prosthesis was abandoned because of the fatal problem of tracheoinnominate artery fistula [14]. This complication was considered to be closely related to the pore size and stiffness of the mesh.
Because the major structural elements (epithelium and cartilage) and the primary function (maintaining airway patency, clearance of sputum) of the trachea are relatively simple, recent attempts to rebuild the trachea have involved biomedical tissue engineering. Through current technology, regeneration of cartilage has experimentally proved effective in the repair of partial defects of the tracheal wall [15]; but this strategy has failed to maintain patency in circumferential tracheal defects of animals, all succumbing to airway obstruction by hyperproliferative granulation tissue [16].
In recent years, using a decellularized tissue-engineered trachea to treat tracheal diseases has accomplished a notable clinical achievement [17, 18]. However, after transplantation, tracheal stenosis appeared at the anastomotic site, which required repeated endoluminal stenting to maintain the patency of the airway lumen. Besides, until clarifying the functions of the seeded cells more clearly, concerns remained about the fate of chondrocytes on the tracheal scaffold. It is unclear whether new chondrogenesis from the seeded chondrocytes would occur [19, 20]. This method still has other drawbacks, such as procedural complexity, donor shortage, etc. The major disadvantage is the need for human donor trachea to prepare the tubular tracheal matrices. Admittedly, this technology is more in the realm of transplantation than in the field of prosthetics.
Meanwhile, researchers remain focused on rebuilding airways by artificial means. Shimizu et al. [21] and Bottema et al. [22] demonstrated that the optimal pore size of the mesh during the wound healing process was about 200–300 μm. Fine Marlex mesh (pore size, 260 μm) belonged to this range, and this mesh had a certain degree of strength to maintain the patency of the artificial trachea [23]. Afterwards, Hirai and colleagues [24] used a fine Marlex mesh conjugated with collagen to repair window defects in tracheas and found that repairing with the collagen-conjugated mesh was superior to that with the mesh without collagen in terms of the wound-healing process.
Okumura and coworkers [4] prepared a straight prosthesis made from collagen-conjugated fine Marlex mesh to replace a 2 cm segment of cervical trachea without omental wrapping and use of a silicone tube as a stent in 13 dogs. All prostheses were completely incorporated by host tracheas, but such short prostheses were inadequate for clinical purposes. Two dogs died of pneumonia about 2 months postoperatively, and three animals showed marked stenosis of prosthetic lumen due to excessive scar tissue.
Teramachi and coworkers further evaluated the merits of omental wrapping [4], using 5 cm lengths of collagen-conjugated prosthetic trachea to reconstruct the intrathoracic tracheas of dogs. A silicone tube was inserted into the lumen of each prosthesis and sutured to the host trachea. Better epithelialization and fewer complications (such as luminal stenosis) were observed in the group undergoing omentopexy (versus controls). However, despite these efforts, more than half of the tracheal lumen was stenotic in two of eight dogs subjected to omentopexy.
Teramachi and associates [5] have also designed a porous-type tracheal prosthesis. After freeze-drying, the collagen sponge-sealed mesh cylinder was heated to 105°C in vacuum for 12 h to produce moderate crosslinking of collagen molecules. This prosthesis (in 5 cm lengths) was used to reconstruct the cervical trachea in 10 mongrel dogs, and silicon tubes again were inserted as stents. One dog died of a postsurgical (week 6) anaesthesia-related incident. Significant circumferential stenosis was identified in three dogs just proximal to the stents, still in place after reconstruction (week 4); and one of these dogs died of suffocation at week 10.
The collagen extracted from porcine skin for prosthesis preparation is an extracellular matrix component with low antigenicity, playing an important role in cellular division, proliferation, differentiation and transformation. It is chiefly composed of Type I (70–80%) and Type III (20–30%) fractions. Type I collagen promotes differentiation of ciliated epithelium, inducing regeneration of airway epithelium. Collagen not only ensures the airtightness of a tracheal prosthesis but also provides a suitable environment for autologous tissue ingrowth. In the weeks following surgery, the collagen of prostheses gradually decomposes and is replaced by autologous tissue. Collagen also provides a substrate for platelet adhesion, culminating in copious release of platelet-derived growth factor to promote cellular proliferation and accelerate healing between host tissues and prosthetic elements [5, 6].
Furthermore, the Marlex mesh tube coated with collagen sponge has been applied for repair of the partial tracheal defect in human cases successfully [7–9]. These patients suffered from tracheal benign stenosis (n = 4) or thyroid cancer with tracheal invasion (n = 3). After resection of the lesions, the artificial material was trimmed and sutured to the defect of the trachea. Epithelium covered the implanted material after 2 months. The longest observation period was 34 months. Endoscopic examination revealed a well-epithelialized luminal surface without any airway obstruction.
The high biocompatibility of collagen-conjugated prostheses has helped overcome potentially fatal outcomes of anastomotic dehiscence and prosthesis dislocation. However, omental wrapping and silicone tube stenting are still injurious to hosts. After participating in the development of a Y-shaped tracheal prosthesis [6, 25], we similarly prepared a straight-tube tracheal prosthesis for use in the present study, which improved on the design of Okumura and colleagues [3]. This 5-cm long artificial trachea without stenting or a pedicle-bearing omental wrapping had been incorporated by host tissue, showing no anastomotic dehiscence or prosthesis dislocation. The prosthesis coated with collagen allowed adjacent connective tissue (including small vessels) ingrowth through the pores of the mesh [3, 24]. Subsequently neo-vascularization of the prosthesis would occur to promote epithelial regeneration. The inner surface of the artificial trachea was covered with the host epithelium and the anastomosis sites were healed with the host tissue [3, 6]. Host dogs could survive as long as the prosthesis maintained patency and avoided anastomotic dehiscence and prosthesis dislocation. In the present experiment, we observed that the dogs could survive for a long time after implantation of artificial tracheas in 5 cm lengths. Our results are still acceptable by comparison (in terms of luminal stenosis), without use of an omental wrap.
Insertion of a silicone tube as part of the surgical protocol is intended to promote clearance of sputum and to prevent infection. However, there are no experimental data to confirm that this step is truly necessary. Because we encountered no sputum retention as a consequence of eliminating such stents, it is our view that insertion of a silicone tube is not warranted in this setting.
As a final point of discussion, Teramachi et al. [5] noted that the collagen sponge is easily detached from the mesh cylinder of the prosthesis during anastomosis. We found this to be problematic as well and handled the prosthesis with greater care. Exuberant proliferations of granulation tissue may occur in places where host tissues fall short in replacing absorbed collagen. In extreme instances, stenosis or even asphyxia may result. We are attempting to improve this technology in future studies, assuring that collagen is adequate for the task through enhanced adherence to the artificial trachea. Thus, the problem of luminal stenosis will hopefully be resolved, without resorting to omental wrapping.
The tracheal prostheses utilized in the present study (∼5 cm in length) were fabricated from collagen-conjugated mesh, imparting greater biocompatibility. Consequently, we investigated this type of tracheal prosthesis to replace segments of cervical trachea without the need of omental wrapping (requiring an additional abdominal procedure) and silicone stenting (subject to later postoperative extraction), which considerably simplified the surgical procedures and eased the host ordeal in turn. This pilot study suggested a possibility of clinical application.
Funding
This work was supported by Shanghai Municipal Science and Technology Commission (grant number 12ZR1406000).
Conflict of interest: none declared.
REFERENCES
Author notes
This experiment was carried out at Zhongshan Hospital, Fudan University, Shanghai, China.