-
PDF
- Split View
-
Views
-
Cite
Cite
Ligia RS Kerr-Pontes, Maurício L Barreto, Clara MN Evangelista, Laura C Rodrigues, Jorg Heukelbach, Hermann Feldmeier, Socioeconomic, environmental, and behavioural risk factors for leprosy in North-east Brazil: results of a case–control study, International Journal of Epidemiology, Volume 35, Issue 4, August 2006, Pages 994–1000, https://doi.org/10.1093/ije/dyl072
Close - Share Icon Share
Abstract
Background Brazil reports almost 80% of all leprosy cases in the Americas. This study aimed to identify socioeconomic, environmental, and behavioural factors associated with risk of leprosy occurrence in the endemic North-eastern region.
Methods A case–control study in four municipalities. Cases: cases of leprosy diagnosed in the previous 2 years, with no other known, current, or past case of leprosy in the household or in the neighbourhood. Controls: individuals presenting for reasons other than skin problems to the health unit where the case was diagnosed and who lived in the same municipality as the case with whom it was matched. For each case four controls were selected. A semi-structured questionnaire was used to collect demographic, socioeconomic, environmental, and behavioural data. A multivariate hierarchical analysis was performed according to a previously defined framework.
Results 226 cases and 857 controls were examined. Low education level, ever having experienced food shortage, bathing weekly in open water bodies (creek, river and/or lake) 10 years previously, and a low frequency of changing bed linen or hammock (≥biweekly) currently were all significantly associated with leprosy. Having a BCG vaccination scar was found to be a highly significant protective factor.
Conclusions Except for BCG vaccination, variables that remained significant in the hierarchical analysis are cultural or linked to poverty. They may act on different levels of the transmission of Mycobacterium leprae and/or the progress from infection to disease. These findings give credit to the hypothesis that person-to-person is not the only form of M. leprae transmission, and that indirect transmission might occur, and other reservoirs should exist outside the human body.
Leprosy is an old disease that continues to be an important public health problem in several developing countries. In over a hundred countries the disease is endemic, and in twelve the prevalence is above the benchmark set by the World Health Organization of 1 new case per 10 000 inhabitants per year.1 Transmission of leprosy is accepted to be primarily person-to-person: the risk of developing leprosy is 5–10 times higher if one member of the family has developed the disease previously2,3 and higher if the primary case has lepromatous leprosy and lower if tuberculoid leprosy.
Although a family contact increases the risk of leprosy, in a typical endemic area the majority of new cases cannot be linked to intra-domiciliary contact with a leprosy patient.2,4 This suggests the existence of unrecognized human-to-human contacts or more intriguing other modes of transmission.5
As Mycobacterium leprae can persist and possibly proliferate in the environment in association with certain plants and animals,6,7 it is conceivable that infection may result through prolonged or repeated exposure to an environmental source containing viable bacilli. This is difficult to investigate experimentally because M. leprae cannot be cultivated in vitro and evidence can only be obtained indirectly through epidemiological studies.
Brazil, India, Nepal, Myanmar, Madagascar, and Mozambique contribute almost 90% to the leprosy cases registered worldwide.8,9 Eighty percentage of all leprosy cases of the Americas occur in Brazil.10 Leprosy is unevenly distributed within Brazil: the North-east Region, the poorest region in the federation, reported 33.5% of newly diagnosed cases (3.2 cases per 10 000 inhabitants) whereas the industrialized South region, one of the richest, reported only 4.1% (0.7 cases per 10 000 inhabitants) in 2002.11 The new case detection rate in the North-east is twice that of the average of the country as a whole and increased over the last decade.12
The study reported here aimed to identify socioeconomic, environmental, and behavioural factors associated with leprosy occurrence in patient with no known leprosy contacts.
Materials and methods
Study area
The study was conducted in four municipalities in the State of Ceará, North-east Brazil. The North-eastern region has a semi-arid climate and regularly suffers from droughts. Half of the population lives in poverty. Social and economic inequalities are important, with 50% of the population earning 16% of the total income of the region and the richest 1% earning 16%.11 Ceará, with a population of ∼7.5 million is in the centre of the North-east region and is one of the three poorest states in the region.11,12 Only 3.7% of the population earns more than US $450 per month, and 28% of the population >15 years of age are illiterate.12
In 2002, 2520 new cases of leprosy were notified in Ceará. As within the state the distribution of leprosy shows a remarkable heterogeneity, we decided to use analytic epidemiology to investigate hitherto unknown risk factors, which might explain the failure of control efforts to reduce the incidence. In a first step, a spatial analysis identified hyperendemic pockets: in a few municipalities the prevalence was astoundingly high, whereas in the majority prevalence and incidence were rather low. The reasons for this aggregation could not be precisely identified, but inequality and uncontrolled urbanization seemed to play an important role.13
Of the 19 municipalities with the highest detection rates four (Juazeiro do Norte, Morada Nova, Sobral e Fortaleza) were selected for inclusion in the study to reflect the physical (climate, elevation, soil type) and socioeconomic diversities of the State of Ceará.
Study population
Cases were selected from patients diagnosed in the previous 2 years in a Primary Health Care Centre through the leprosy registry of Ceará's Ministry of Health. Study cases were selected when they returned to the outpatient clinics for routine monitoring. Leprosy diagnosis was based on the presence of one or more of the following criteria: (i) typical skin lesion with loss of sensitivity; (ii) enlargement of one of the major nerves with loss of sensitivity; (iii) positive skin smear for M. leprae, examined by a trained health professional in a state reference laboratory. Based on information registered by the physician in charge of leprosy cases diagnosis, cases were grouped according to the Ridley & Jopling classification.14 Age 18 years old or less, existence of another case of leprosy in the same household, in the near neighbourhood, or within kinship were exclusion criteria.
Controls were individuals presenting to the same health unit (as the leprosy case) for reasons other than skin problems and who lived in the same municipality. Age 18 years old or less, report of a case of leprosy in the same household, in the near neighbourhood, or within kinship were exclusion criteria. Four controls were selected for each case.
Sample size
The sample size was calculated to allow an odds ratio (OR) of 1.7 for an assumed frequency of exposure of 30% (based on a pilot study), with a confidence of 95% and a power of the test of 80%. This resulted in an estimated sample size of 200 cases and 800 controls, approximately.
Data collection
A pre-tested semi-structured questionnaire was used to collect demographic, socioeconomic, environmental, and behavioural data from cases and controls. Trained health professionals were responsible for interrogating cases and controls at the health units. To account for the long and variable incubation period, cases and controls were asked for risk factors recently and 10 years previously. All patients diagnosed between March and August 2002 as a new case of leprosy and/or in treatment for leprosy (any classification) that fitted the inclusion criteria for a case were interviewed as well as the controls.
Data analysis
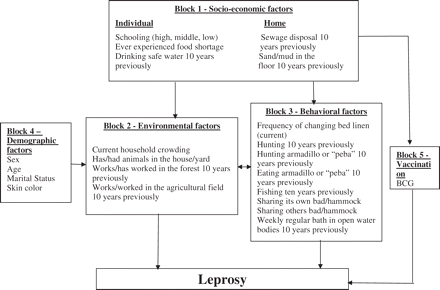
Variables were first analysed in a bivariate manner to identify the variables to be included in the unconditional logistic regression. In a second step a multivariate hierarchical analysis was performed according to a previously defined framework (Figure 1). The framework comprised five blocks, each containing several variables: Block 1—socioeconomic (schooling, experience of food shortage at any time in life, access to safe drinking treated water, sewage and type of floor in the household 10 years previously); Block 2—environmental (household crowding, having or having had animals in the house/yard 10 years previously; working/have ever worked in agricultural field 10 years previously, working/have ever worked in the forest); Block 3—behavioural (current frequency of changing bed linen; hunting and fishing 10 years previously, hunting armadillo or ‘peba’ (Euphractus sexcinctus) 10 years previously, eating armadillo or ‘peba’; sharing own bed/hammock (current); sharing others bed/hammock (current); weekly regular bath in open water bodies—like creek, river, and/or lake—10 years previously); Block 4—demographic (sex, age, marital status, and skin colour); and Block 5—Vaccination (presence of a BCG scar).
Framework for socio-economic, environmental, and behavioural factors in leprosy determination
The adjusted analysis was performed in two steps. In the first one, ORs for each variable were calculated adjusting for all variables in the block. In the second step, the socioeconomic block was adjusted by statistically significant variables of the remaining blocks (2, 3, 4, and 5) as they were considered to have the same level of causation in the model. Cluster effect for municipality was taken into consideration. To be entered into the logistic model a significance of P < 0.25 was required and to remain in the model a significance of P < 0.05. Confounding and interaction between variables (including differences of effects in the four municipalities) were investigated. We used frequency matching for sex and age, and therefore kept age and sex in the multivariate models.
Ethical consideration
The study was approved by the Ethical Committee of Federal University of Ceará. Cases and controls were only included after written informed consent was obtained and they were reassured that non-participation would not affect their treatment.
Results
Information was obtained from 226 cases and 857 controls. Fifteen per cent of cases were indeterminate, 40% tuberculoid, 26% borderline, and 19% lepromatous (Table 1). Within these groups, the male to female ratio ranged from 0.56 to 1.1. Table 2 summarizes the results of the bivariate analysis. Of the socioeconomic variables a low education level, experienced food shortage at any time in life, and living (10 years previously) in a house with sand/mud floor were statistically associated with an increased risk of leprosy. In the demographic block, there were no significant gender, age, colour, or marital status differences. None of the environmental and demographic variables was associated with an increased risk of leprosy.
Demographic and clinical characteristics of leprosy cases and controls
. | Juazeiro do Norte . | Morada Nova . | Sobral . | Fortaleza . |
|---|---|---|---|---|
| Number of leprosy cases | 122 | 39 | 22 | 39 |
| Borderline | 27 | 5 | 11 | 15 |
| Lepromatous | 23 | 12 | 3 | 5 |
| Tuberculoid | 59 | 17 | 5 | 7 |
| Indeterminate | 13 | 5 | 3 | 12 |
| Age (median, range) | 51 (20–78) | 50 (20–87) | 51 (20–77) | 38 (20–79) |
| Male/female | 64/58 | 19/20 | 10/12 | 14/25 |
| Number of controls | 517 | 121 | 74 | 141 |
| Age (median, range) | 48 (19–87) | 34 (20–72) | 30 (18–72) | 35 (20–76) |
| Male/female | 223/294 | 50/71 | 21/53 | 51/90 |
. | Juazeiro do Norte . | Morada Nova . | Sobral . | Fortaleza . |
|---|---|---|---|---|
| Number of leprosy cases | 122 | 39 | 22 | 39 |
| Borderline | 27 | 5 | 11 | 15 |
| Lepromatous | 23 | 12 | 3 | 5 |
| Tuberculoid | 59 | 17 | 5 | 7 |
| Indeterminate | 13 | 5 | 3 | 12 |
| Age (median, range) | 51 (20–78) | 50 (20–87) | 51 (20–77) | 38 (20–79) |
| Male/female | 64/58 | 19/20 | 10/12 | 14/25 |
| Number of controls | 517 | 121 | 74 | 141 |
| Age (median, range) | 48 (19–87) | 34 (20–72) | 30 (18–72) | 35 (20–76) |
| Male/female | 223/294 | 50/71 | 21/53 | 51/90 |
Demographic and clinical characteristics of leprosy cases and controls
. | Juazeiro do Norte . | Morada Nova . | Sobral . | Fortaleza . |
|---|---|---|---|---|
| Number of leprosy cases | 122 | 39 | 22 | 39 |
| Borderline | 27 | 5 | 11 | 15 |
| Lepromatous | 23 | 12 | 3 | 5 |
| Tuberculoid | 59 | 17 | 5 | 7 |
| Indeterminate | 13 | 5 | 3 | 12 |
| Age (median, range) | 51 (20–78) | 50 (20–87) | 51 (20–77) | 38 (20–79) |
| Male/female | 64/58 | 19/20 | 10/12 | 14/25 |
| Number of controls | 517 | 121 | 74 | 141 |
| Age (median, range) | 48 (19–87) | 34 (20–72) | 30 (18–72) | 35 (20–76) |
| Male/female | 223/294 | 50/71 | 21/53 | 51/90 |
. | Juazeiro do Norte . | Morada Nova . | Sobral . | Fortaleza . |
|---|---|---|---|---|
| Number of leprosy cases | 122 | 39 | 22 | 39 |
| Borderline | 27 | 5 | 11 | 15 |
| Lepromatous | 23 | 12 | 3 | 5 |
| Tuberculoid | 59 | 17 | 5 | 7 |
| Indeterminate | 13 | 5 | 3 | 12 |
| Age (median, range) | 51 (20–78) | 50 (20–87) | 51 (20–77) | 38 (20–79) |
| Male/female | 64/58 | 19/20 | 10/12 | 14/25 |
| Number of controls | 517 | 121 | 74 | 141 |
| Age (median, range) | 48 (19–87) | 34 (20–72) | 30 (18–72) | 35 (20–76) |
| Male/female | 223/294 | 50/71 | 21/53 | 51/90 |
Bivariate analysis of demographic, socioeconomic, behavioural, and environmental variables with leprosy
| Factors . | Case no. (%) . | Control no. (%) . | Crude odds ratio (CI)a . | |||
|---|---|---|---|---|---|---|
| Socioeconomic variables | ||||||
| Schooling | ||||||
| High | 40 (18) | 244 (28) | 1 | |||
| Middle | 56 (25) | 226 (26) | 1.51 (0.93–2.47) | |||
| Low | 130 (58) | 387 (45) | 2.05 (1.29–3.27) | |||
| Food shortage at any time in life | ||||||
| Never experienced | 161 (72) | 687 (81) | 1 | |||
| Experienced | 63 (28) | 163 (19) | 1.65 (1.11–2.42) | |||
| Access to safe drinking water 10 years previously | ||||||
| Yes | 133 (59) | 546 (65) | 1 | |||
| No | 91 (41) | 298 (35) | 1.17 (0.96–1.43) | |||
| Sewage disposal 10 years previously | ||||||
| Yes | 180 (81) | 741 (87) | 1 | |||
| No | 41 (19) | 111 (13) | 1.44 (0.95–2.80) | |||
| Sand/mud in the floor 10 years previously | ||||||
| No | 182 (81) | 737 (86) | 1 | |||
| Yes | 44 (20) | 119 (14) | 1.46 (1.04–2.06) | |||
| Environmental variables (a) | ||||||
| Household crowding (currently) | ||||||
| 0–3 persons per room | 200 (89) | 782 (92) | 1 | |||
| 4 or + persons per room | 26 (12) | 71 (8) | 1.43 (0.64–3.20) | |||
| Has/had animals in the house/yard 10 years previously | ||||||
| No | 39 (17) | 203 (24) | 1 | |||
| Yes | 184 (83) | 649 (77) | 1.48 (0.77–2.86) | |||
| Works/worked in forest 10 years previously | ||||||
| No | 148 (69) | 647 (76) | 1 | |||
| Yes | 68 (31) | 200 (24) | 1.43 (0.90–2.29) | |||
| Works/worked in agricultural field 10 years previously | ||||||
| No | 81 (36) | 392 (46) | 1 | |||
| Yes | 144 (64) | 463 (54) | 1.48 (0.79–2.77) | |||
| Behavioural variables | ||||||
| Frequency of changing bed linen (current) | ||||||
| <Biweekly | 132 (58) | 609 (72) | 1 | |||
| ≥Biweekly | 94 (42) | 242 (28) | 1.79 (1.32–2.43) | |||
| Hunting 10 years previously | ||||||
| No | 196 (88) | 788 (93) | 1 | |||
| Yes | 26 (12) | 62 (7) | 1.69 (1.04–2.74) | |||
| Hunting armadillo or ‘peba’ 10 years previously | ||||||
| No | 217 (96) | 833 (97) | 1 | |||
| Yes | 9 (4) | 24 (3) | 1.42 (1.12–1.79) | |||
| Eating armadillo or ‘peba’ 10 years previously | ||||||
| No | 83 (37) | 338 (40) | 1 | |||
| Yes | 141 (63) | 504 (60) | 0.83 (0.65–1.05) | |||
| Fishing 10 years previously | ||||||
| Never | 183 (82) | 730 (86) | 1 | |||
| Seldomly | 31 (14) | 107 (13) | 1.16 (0.92–1.45) | |||
| Regularly | 8 (4) | 13 (2) | 2.45 (1.46–4.12) | |||
| Sharing its own bed/hammock with others (current) | ||||||
| Yes | 100 (44) | 428 (50) | 1 | |||
| No | 125 (56) | 426 (50) | 1.29 (0.93–1.61) | |||
| Sharing others bed/hammock with others (current) | ||||||
| Yes | 131 (58) | 526 (62) | 1 | |||
| No | 95 (42) | 322 (38) | 1.17 (0.60–2.30) | |||
| Weekly regular bath in open water bodies (creek, river and/or lake) 10 years previously | ||||||
| No | 188 (83) | 770 (90) | 1 | |||
| Yes | 38 (17) | 87 (10) | 1.79 (1.18–2.70) | |||
| Demographic variables | ||||||
| Sex | ||||||
| Male | 108 (48) | 348 (41) | 1 | |||
| Female | 118 (52) | 509 (59) | 0.84 (0.68–1.04) | |||
| Age (years) | ||||||
| <30 | 44 (20) | 228 (27) | 1 | |||
| 30–39 | 33 (15) | 167 (19) | 1.02 (0.72–1.45) | |||
| ≥40 | 149 (66) | 462 (54) | 1.67 (0.77–3.64) | |||
| Skin colour | ||||||
| White | 76 (34) | 404 (47) | 1 | |||
| Brown/black | 148 (66) | 450 (53) | 1.88 (0.99–3.56) | |||
| Marital status | ||||||
| Not married | 78 (35) | 298 (35) | 1 | |||
| Married | 144 (65) | 552 (65) | 0.95 (0.84–1.06) | |||
| Vaccination | ||||||
| BCG scar | ||||||
| No | 153 (69) | 403 (47) | 1 | |||
| Yes | 70 (31) | 451 (53) | 0.41 (0.30–0.56) | |||
| Factors . | Case no. (%) . | Control no. (%) . | Crude odds ratio (CI)a . | |||
|---|---|---|---|---|---|---|
| Socioeconomic variables | ||||||
| Schooling | ||||||
| High | 40 (18) | 244 (28) | 1 | |||
| Middle | 56 (25) | 226 (26) | 1.51 (0.93–2.47) | |||
| Low | 130 (58) | 387 (45) | 2.05 (1.29–3.27) | |||
| Food shortage at any time in life | ||||||
| Never experienced | 161 (72) | 687 (81) | 1 | |||
| Experienced | 63 (28) | 163 (19) | 1.65 (1.11–2.42) | |||
| Access to safe drinking water 10 years previously | ||||||
| Yes | 133 (59) | 546 (65) | 1 | |||
| No | 91 (41) | 298 (35) | 1.17 (0.96–1.43) | |||
| Sewage disposal 10 years previously | ||||||
| Yes | 180 (81) | 741 (87) | 1 | |||
| No | 41 (19) | 111 (13) | 1.44 (0.95–2.80) | |||
| Sand/mud in the floor 10 years previously | ||||||
| No | 182 (81) | 737 (86) | 1 | |||
| Yes | 44 (20) | 119 (14) | 1.46 (1.04–2.06) | |||
| Environmental variables (a) | ||||||
| Household crowding (currently) | ||||||
| 0–3 persons per room | 200 (89) | 782 (92) | 1 | |||
| 4 or + persons per room | 26 (12) | 71 (8) | 1.43 (0.64–3.20) | |||
| Has/had animals in the house/yard 10 years previously | ||||||
| No | 39 (17) | 203 (24) | 1 | |||
| Yes | 184 (83) | 649 (77) | 1.48 (0.77–2.86) | |||
| Works/worked in forest 10 years previously | ||||||
| No | 148 (69) | 647 (76) | 1 | |||
| Yes | 68 (31) | 200 (24) | 1.43 (0.90–2.29) | |||
| Works/worked in agricultural field 10 years previously | ||||||
| No | 81 (36) | 392 (46) | 1 | |||
| Yes | 144 (64) | 463 (54) | 1.48 (0.79–2.77) | |||
| Behavioural variables | ||||||
| Frequency of changing bed linen (current) | ||||||
| <Biweekly | 132 (58) | 609 (72) | 1 | |||
| ≥Biweekly | 94 (42) | 242 (28) | 1.79 (1.32–2.43) | |||
| Hunting 10 years previously | ||||||
| No | 196 (88) | 788 (93) | 1 | |||
| Yes | 26 (12) | 62 (7) | 1.69 (1.04–2.74) | |||
| Hunting armadillo or ‘peba’ 10 years previously | ||||||
| No | 217 (96) | 833 (97) | 1 | |||
| Yes | 9 (4) | 24 (3) | 1.42 (1.12–1.79) | |||
| Eating armadillo or ‘peba’ 10 years previously | ||||||
| No | 83 (37) | 338 (40) | 1 | |||
| Yes | 141 (63) | 504 (60) | 0.83 (0.65–1.05) | |||
| Fishing 10 years previously | ||||||
| Never | 183 (82) | 730 (86) | 1 | |||
| Seldomly | 31 (14) | 107 (13) | 1.16 (0.92–1.45) | |||
| Regularly | 8 (4) | 13 (2) | 2.45 (1.46–4.12) | |||
| Sharing its own bed/hammock with others (current) | ||||||
| Yes | 100 (44) | 428 (50) | 1 | |||
| No | 125 (56) | 426 (50) | 1.29 (0.93–1.61) | |||
| Sharing others bed/hammock with others (current) | ||||||
| Yes | 131 (58) | 526 (62) | 1 | |||
| No | 95 (42) | 322 (38) | 1.17 (0.60–2.30) | |||
| Weekly regular bath in open water bodies (creek, river and/or lake) 10 years previously | ||||||
| No | 188 (83) | 770 (90) | 1 | |||
| Yes | 38 (17) | 87 (10) | 1.79 (1.18–2.70) | |||
| Demographic variables | ||||||
| Sex | ||||||
| Male | 108 (48) | 348 (41) | 1 | |||
| Female | 118 (52) | 509 (59) | 0.84 (0.68–1.04) | |||
| Age (years) | ||||||
| <30 | 44 (20) | 228 (27) | 1 | |||
| 30–39 | 33 (15) | 167 (19) | 1.02 (0.72–1.45) | |||
| ≥40 | 149 (66) | 462 (54) | 1.67 (0.77–3.64) | |||
| Skin colour | ||||||
| White | 76 (34) | 404 (47) | 1 | |||
| Brown/black | 148 (66) | 450 (53) | 1.88 (0.99–3.56) | |||
| Marital status | ||||||
| Not married | 78 (35) | 298 (35) | 1 | |||
| Married | 144 (65) | 552 (65) | 0.95 (0.84–1.06) | |||
| Vaccination | ||||||
| BCG scar | ||||||
| No | 153 (69) | 403 (47) | 1 | |||
| Yes | 70 (31) | 451 (53) | 0.41 (0.30–0.56) | |||
OR was calculated taking into account cluster effect of municipalities.
Bivariate analysis of demographic, socioeconomic, behavioural, and environmental variables with leprosy
| Factors . | Case no. (%) . | Control no. (%) . | Crude odds ratio (CI)a . | |||
|---|---|---|---|---|---|---|
| Socioeconomic variables | ||||||
| Schooling | ||||||
| High | 40 (18) | 244 (28) | 1 | |||
| Middle | 56 (25) | 226 (26) | 1.51 (0.93–2.47) | |||
| Low | 130 (58) | 387 (45) | 2.05 (1.29–3.27) | |||
| Food shortage at any time in life | ||||||
| Never experienced | 161 (72) | 687 (81) | 1 | |||
| Experienced | 63 (28) | 163 (19) | 1.65 (1.11–2.42) | |||
| Access to safe drinking water 10 years previously | ||||||
| Yes | 133 (59) | 546 (65) | 1 | |||
| No | 91 (41) | 298 (35) | 1.17 (0.96–1.43) | |||
| Sewage disposal 10 years previously | ||||||
| Yes | 180 (81) | 741 (87) | 1 | |||
| No | 41 (19) | 111 (13) | 1.44 (0.95–2.80) | |||
| Sand/mud in the floor 10 years previously | ||||||
| No | 182 (81) | 737 (86) | 1 | |||
| Yes | 44 (20) | 119 (14) | 1.46 (1.04–2.06) | |||
| Environmental variables (a) | ||||||
| Household crowding (currently) | ||||||
| 0–3 persons per room | 200 (89) | 782 (92) | 1 | |||
| 4 or + persons per room | 26 (12) | 71 (8) | 1.43 (0.64–3.20) | |||
| Has/had animals in the house/yard 10 years previously | ||||||
| No | 39 (17) | 203 (24) | 1 | |||
| Yes | 184 (83) | 649 (77) | 1.48 (0.77–2.86) | |||
| Works/worked in forest 10 years previously | ||||||
| No | 148 (69) | 647 (76) | 1 | |||
| Yes | 68 (31) | 200 (24) | 1.43 (0.90–2.29) | |||
| Works/worked in agricultural field 10 years previously | ||||||
| No | 81 (36) | 392 (46) | 1 | |||
| Yes | 144 (64) | 463 (54) | 1.48 (0.79–2.77) | |||
| Behavioural variables | ||||||
| Frequency of changing bed linen (current) | ||||||
| <Biweekly | 132 (58) | 609 (72) | 1 | |||
| ≥Biweekly | 94 (42) | 242 (28) | 1.79 (1.32–2.43) | |||
| Hunting 10 years previously | ||||||
| No | 196 (88) | 788 (93) | 1 | |||
| Yes | 26 (12) | 62 (7) | 1.69 (1.04–2.74) | |||
| Hunting armadillo or ‘peba’ 10 years previously | ||||||
| No | 217 (96) | 833 (97) | 1 | |||
| Yes | 9 (4) | 24 (3) | 1.42 (1.12–1.79) | |||
| Eating armadillo or ‘peba’ 10 years previously | ||||||
| No | 83 (37) | 338 (40) | 1 | |||
| Yes | 141 (63) | 504 (60) | 0.83 (0.65–1.05) | |||
| Fishing 10 years previously | ||||||
| Never | 183 (82) | 730 (86) | 1 | |||
| Seldomly | 31 (14) | 107 (13) | 1.16 (0.92–1.45) | |||
| Regularly | 8 (4) | 13 (2) | 2.45 (1.46–4.12) | |||
| Sharing its own bed/hammock with others (current) | ||||||
| Yes | 100 (44) | 428 (50) | 1 | |||
| No | 125 (56) | 426 (50) | 1.29 (0.93–1.61) | |||
| Sharing others bed/hammock with others (current) | ||||||
| Yes | 131 (58) | 526 (62) | 1 | |||
| No | 95 (42) | 322 (38) | 1.17 (0.60–2.30) | |||
| Weekly regular bath in open water bodies (creek, river and/or lake) 10 years previously | ||||||
| No | 188 (83) | 770 (90) | 1 | |||
| Yes | 38 (17) | 87 (10) | 1.79 (1.18–2.70) | |||
| Demographic variables | ||||||
| Sex | ||||||
| Male | 108 (48) | 348 (41) | 1 | |||
| Female | 118 (52) | 509 (59) | 0.84 (0.68–1.04) | |||
| Age (years) | ||||||
| <30 | 44 (20) | 228 (27) | 1 | |||
| 30–39 | 33 (15) | 167 (19) | 1.02 (0.72–1.45) | |||
| ≥40 | 149 (66) | 462 (54) | 1.67 (0.77–3.64) | |||
| Skin colour | ||||||
| White | 76 (34) | 404 (47) | 1 | |||
| Brown/black | 148 (66) | 450 (53) | 1.88 (0.99–3.56) | |||
| Marital status | ||||||
| Not married | 78 (35) | 298 (35) | 1 | |||
| Married | 144 (65) | 552 (65) | 0.95 (0.84–1.06) | |||
| Vaccination | ||||||
| BCG scar | ||||||
| No | 153 (69) | 403 (47) | 1 | |||
| Yes | 70 (31) | 451 (53) | 0.41 (0.30–0.56) | |||
| Factors . | Case no. (%) . | Control no. (%) . | Crude odds ratio (CI)a . | |||
|---|---|---|---|---|---|---|
| Socioeconomic variables | ||||||
| Schooling | ||||||
| High | 40 (18) | 244 (28) | 1 | |||
| Middle | 56 (25) | 226 (26) | 1.51 (0.93–2.47) | |||
| Low | 130 (58) | 387 (45) | 2.05 (1.29–3.27) | |||
| Food shortage at any time in life | ||||||
| Never experienced | 161 (72) | 687 (81) | 1 | |||
| Experienced | 63 (28) | 163 (19) | 1.65 (1.11–2.42) | |||
| Access to safe drinking water 10 years previously | ||||||
| Yes | 133 (59) | 546 (65) | 1 | |||
| No | 91 (41) | 298 (35) | 1.17 (0.96–1.43) | |||
| Sewage disposal 10 years previously | ||||||
| Yes | 180 (81) | 741 (87) | 1 | |||
| No | 41 (19) | 111 (13) | 1.44 (0.95–2.80) | |||
| Sand/mud in the floor 10 years previously | ||||||
| No | 182 (81) | 737 (86) | 1 | |||
| Yes | 44 (20) | 119 (14) | 1.46 (1.04–2.06) | |||
| Environmental variables (a) | ||||||
| Household crowding (currently) | ||||||
| 0–3 persons per room | 200 (89) | 782 (92) | 1 | |||
| 4 or + persons per room | 26 (12) | 71 (8) | 1.43 (0.64–3.20) | |||
| Has/had animals in the house/yard 10 years previously | ||||||
| No | 39 (17) | 203 (24) | 1 | |||
| Yes | 184 (83) | 649 (77) | 1.48 (0.77–2.86) | |||
| Works/worked in forest 10 years previously | ||||||
| No | 148 (69) | 647 (76) | 1 | |||
| Yes | 68 (31) | 200 (24) | 1.43 (0.90–2.29) | |||
| Works/worked in agricultural field 10 years previously | ||||||
| No | 81 (36) | 392 (46) | 1 | |||
| Yes | 144 (64) | 463 (54) | 1.48 (0.79–2.77) | |||
| Behavioural variables | ||||||
| Frequency of changing bed linen (current) | ||||||
| <Biweekly | 132 (58) | 609 (72) | 1 | |||
| ≥Biweekly | 94 (42) | 242 (28) | 1.79 (1.32–2.43) | |||
| Hunting 10 years previously | ||||||
| No | 196 (88) | 788 (93) | 1 | |||
| Yes | 26 (12) | 62 (7) | 1.69 (1.04–2.74) | |||
| Hunting armadillo or ‘peba’ 10 years previously | ||||||
| No | 217 (96) | 833 (97) | 1 | |||
| Yes | 9 (4) | 24 (3) | 1.42 (1.12–1.79) | |||
| Eating armadillo or ‘peba’ 10 years previously | ||||||
| No | 83 (37) | 338 (40) | 1 | |||
| Yes | 141 (63) | 504 (60) | 0.83 (0.65–1.05) | |||
| Fishing 10 years previously | ||||||
| Never | 183 (82) | 730 (86) | 1 | |||
| Seldomly | 31 (14) | 107 (13) | 1.16 (0.92–1.45) | |||
| Regularly | 8 (4) | 13 (2) | 2.45 (1.46–4.12) | |||
| Sharing its own bed/hammock with others (current) | ||||||
| Yes | 100 (44) | 428 (50) | 1 | |||
| No | 125 (56) | 426 (50) | 1.29 (0.93–1.61) | |||
| Sharing others bed/hammock with others (current) | ||||||
| Yes | 131 (58) | 526 (62) | 1 | |||
| No | 95 (42) | 322 (38) | 1.17 (0.60–2.30) | |||
| Weekly regular bath in open water bodies (creek, river and/or lake) 10 years previously | ||||||
| No | 188 (83) | 770 (90) | 1 | |||
| Yes | 38 (17) | 87 (10) | 1.79 (1.18–2.70) | |||
| Demographic variables | ||||||
| Sex | ||||||
| Male | 108 (48) | 348 (41) | 1 | |||
| Female | 118 (52) | 509 (59) | 0.84 (0.68–1.04) | |||
| Age (years) | ||||||
| <30 | 44 (20) | 228 (27) | 1 | |||
| 30–39 | 33 (15) | 167 (19) | 1.02 (0.72–1.45) | |||
| ≥40 | 149 (66) | 462 (54) | 1.67 (0.77–3.64) | |||
| Skin colour | ||||||
| White | 76 (34) | 404 (47) | 1 | |||
| Brown/black | 148 (66) | 450 (53) | 1.88 (0.99–3.56) | |||
| Marital status | ||||||
| Not married | 78 (35) | 298 (35) | 1 | |||
| Married | 144 (65) | 552 (65) | 0.95 (0.84–1.06) | |||
| Vaccination | ||||||
| BCG scar | ||||||
| No | 153 (69) | 403 (47) | 1 | |||
| Yes | 70 (31) | 451 (53) | 0.41 (0.30–0.56) | |||
OR was calculated taking into account cluster effect of municipalities.
Five out of eight variables in the behavioural block were associated with increased risk of leprosy: low frequency of changing bed linen, hunting 10 years previously, hunting armadillo or ‘peba’ 10 years previously, fishing 10 years previously, weekly regular bath in open water bodies, i.e. creek, river and/or lake, 10 years previously. The presence of a BCG scar offered a statistically highly significant protection against leprosy.
The results of the multivariate hierarchical analysis are summarized in Table 3. Frequency matching did not result in similar proportion by sex. Controlling for sex was done in the multivariate model. After the introduction of the five blocks of variables only those statistically significantly associated with leprosy remained in the model. A low education level (OR = 1.87; 95% CI 1.29–2.74), experienced food shortage at any time in life (OR = 1.54; 95% CI 1.45–1.63), weekly regular bath in open water bodies 10 years previously (OR = 1.77; 95% CI 1.12–2.81), and low frequency of changing bed linen (OR = 1.81; 95% CI 1.30–2.52) were all significantly associated with leprosy. In addition, the presence of BCG vaccination scar remained protective (OR = 0.48; 95% CI 0.33–0.70), corresponding to a vaccine effectiveness of 52%.
Results of the multivariate hierarchical analysis
| Variables . | Socioeconomic block . | Behavioural block . | Vaccination block . |
|---|---|---|---|
| High education | 1 | ||
| Middle education | 1.50 (0.91–2.50) | ||
| Low education | 1.87 (1.29–2.74) | ||
| Experienced food shortage at any time in life | 1.54 (1.45–1.63) | ||
| Weekly regular bath in open water bodies | 1.77 (1.12–2.81) | ||
| Low frequency of changing bed linen or hammock (>biweekly) | 1.81(1.30–2.52) | ||
| Sex—Female | 0.97 (0.70–1.34) | ||
| Age | 1.01 (1.00–1.02) | ||
| BCG scar | 0.48 (0.33–0.70) |
| Variables . | Socioeconomic block . | Behavioural block . | Vaccination block . |
|---|---|---|---|
| High education | 1 | ||
| Middle education | 1.50 (0.91–2.50) | ||
| Low education | 1.87 (1.29–2.74) | ||
| Experienced food shortage at any time in life | 1.54 (1.45–1.63) | ||
| Weekly regular bath in open water bodies | 1.77 (1.12–2.81) | ||
| Low frequency of changing bed linen or hammock (>biweekly) | 1.81(1.30–2.52) | ||
| Sex—Female | 0.97 (0.70–1.34) | ||
| Age | 1.01 (1.00–1.02) | ||
| BCG scar | 0.48 (0.33–0.70) |
Data indicate ORs and 95% CIs.
Results of the multivariate hierarchical analysis
| Variables . | Socioeconomic block . | Behavioural block . | Vaccination block . |
|---|---|---|---|
| High education | 1 | ||
| Middle education | 1.50 (0.91–2.50) | ||
| Low education | 1.87 (1.29–2.74) | ||
| Experienced food shortage at any time in life | 1.54 (1.45–1.63) | ||
| Weekly regular bath in open water bodies | 1.77 (1.12–2.81) | ||
| Low frequency of changing bed linen or hammock (>biweekly) | 1.81(1.30–2.52) | ||
| Sex—Female | 0.97 (0.70–1.34) | ||
| Age | 1.01 (1.00–1.02) | ||
| BCG scar | 0.48 (0.33–0.70) |
| Variables . | Socioeconomic block . | Behavioural block . | Vaccination block . |
|---|---|---|---|
| High education | 1 | ||
| Middle education | 1.50 (0.91–2.50) | ||
| Low education | 1.87 (1.29–2.74) | ||
| Experienced food shortage at any time in life | 1.54 (1.45–1.63) | ||
| Weekly regular bath in open water bodies | 1.77 (1.12–2.81) | ||
| Low frequency of changing bed linen or hammock (>biweekly) | 1.81(1.30–2.52) | ||
| Sex—Female | 0.97 (0.70–1.34) | ||
| Age | 1.01 (1.00–1.02) | ||
| BCG scar | 0.48 (0.33–0.70) |
Data indicate ORs and 95% CIs.
Discussion
In this study we defined a framework for the multivariate hierarchical analysis in order to access the independent effect of the variables. Clearly, all variables that remained significant in the multivariate logistic model are in one way or another linked to poverty. Although it is well established that leprosy is associated with poverty, it is important to elucidate aspects of poverty that may enhance the risk of the transmission of M. leprae and/or facilitate the progress from infection to disease.
The significant variables in the final model were a low education level, the experience of food shortage at any time in life, frequent contact with natural water bodies 10 years previously and an infrequent change of bed linen. An association between low level of school achievements and the incidence of leprosy was also demonstrated in a study in Malawi.15 Education is difficult to interpret at a biological level, as those with a low level of education usually come from the lowest income stratum of a population and, therefore, share many other health hazards, including lack of health education and access to health care. We consider low education as a distant determinant of leprosy. The variable remained in the final analysis because we used a hierarchical model to access its independent effect.
Food shortage leading to hunger is a typical characteristic of low-income households. This factor could be more directly related to leprosy since individuals who suffered from hunger at least once during the last 10 years are likely to have experienced nutritional deficiencies in previous periods of their life. It is conceivable that inadequate nutrition weakens the immune competence against infection and, thereby, the infection with M. leprae.16 Alternatively, this variable could represent a marker for other health hazards associated with extreme poverty such as risky behaviour to increasing exposure.
The low frequency of changing bed linen is related to water shortage, poverty, and hygiene. Personal observations in the study area indicate that even the poorest households are kept clean and that inappropriate hygiene is mainly the consequence of water shortage that is much more frequent in the poorest areas (Feldmeier, unpublished observation 2001). If water is limited the person responsible for household chores (usually the mother) may refrain from frequently changing bed-linen; or irregular change of bed-linen may be a behavioural characteristic linked to inappropriate hygiene perception. M. leprae can survive out of the human body for several months even under unfavourable conditions.7 It is possible that this behaviour could maintain the M. leprae in the bed or hammock and facilitate longer contact and transmission to the user.
Water shortage is frequent in semi-arid regions. When it happens, people tend to concentrate around some remaining source of water but they still live far from each other in the rural area. Therefore, for some decades now the lack of governmental support has driven rural populations to migrate to suburbs of more developed cities when there is a drought in the state and this has been shown previously to be associated with leprosy.13
Another variable with a strong association with leprosy was frequent contact with water bodies such as creeks, rivers, ponds, or lakes for recreational activities 10 years previously. In the semi-arid climate of Ceará, creeks and rivers have running water only during the rainy season (3–5 months of the year), and when precipitation stops pools of stagnant water remain or are dug by the population and become a habitat for a variety of plants and small animals. Similarly, ponds and lakes transform into swamps covered thickly with vegetation in which small pools of water remain. All these sources of water are used by people for recreation and, if households have no access to piped water or a well, for domestic purposes, too.
It is known that viable M. leprae may persist and proliferate in water plants such as Sphagnum species even in cold-climate countries6 and water has been repeatedly suggested as a reservoir for M. leprae.17 Interestingly, water has been considered a putative source of infection with M. leprae already in the early days of leprology. Hansen and Looft18 observed that in Norway—where the West Coast was a hyperendemic area during the 19th century—leprosy lesions were commonly located at the feet and the lower legs. In those times many people walked barefooted (at least during summer) and had to cross rivers and swamps to reach their fields or neighbouring villages. According to Hansen and Looft sores acquired when walking barefooted facilitated the infection with M. leprae in a similar way to that proposed for M. ulcerans today. Before our study, Matsuoka et al.19 had added evidence to this hypothesis; by using M. leprae-specific DNA probes, he showed that in Indonesia the prevalence of leprosy among individuals who used water sources containing M. leprae for bathing and washing clothes or dishes was significantly higher than that among individuals who used water free of M. leprae.
It is an ancillary finding of this study that individuals with a successful BCG vaccination (as indicated by the typical scar) were protected against leprosy (OR = 0.48; 95% CI 0.33–0.70). This observation confirms previous findings suggesting that BCG vaccination partly protects against the development of leprosy.20
Variables reflecting risk factors for person-to-person transmission—such as crowding or sharing the bed or hammock with other household members—did not show a significant association with leprosy; this is probably because cases with known leprosy contact were excluded from the study. Also, in spite of contact with armadillo having been described as a possible source of leprosy transmission in some regions of North America,21–23 we did not find it as a risk factor in our study.
It is a characteristic of leprosy that it is virtually impossible to precisely assess time and duration of exposure and the onset of an infection. It is, therefore, an intrinsic weakness of any epidemiological approach that owing to the long and variable incubation period risk factors have to be looked for that may or may not have been present 10 and more years previously. This increases the recall bias considerably and makes the identification of temporary behavioural characteristics doubtful. We took recall bias into account when we developed the questionnaire and pre-tested it in two of the four study areas and we limited questions to those circumstances that presumably remain in memory such as having experienced hunger at least once in life (rather than asking about food or whether natural habits in the family) or whether natural water bodies were used for recreational activities (instead of asking which type of water was used for domestic and which for recreational activities). Similarly, patients and controls knew very well whether there had been a bathroom in their house 10 years previously, but had difficulties remembering exactly where water had come from for domestic activities during the different periods of the year.
In conclusion, the results of our case–control study show that certain socioeconomic, environmental, and behavioural risk factors exist, which favour the occurrence of leprosy in an endemic area and could be targeted in control measures encompassing more than the correct implementation of multidrug therapy. The observation that frequent contact with natural water bodies is a risk factor for leprosy independent of other behavioural and socioeconomic variables make stronger the notion that water or wet soil may act as a reservoir for M. leprae.
Brazil reports almost 80% of the leprosy cases in the Americas.
Many of the new cases cannot be linked to intra-domiciliary contact with a leprosy patient suggesting the existence of hitherto unknown factors involved in transmission.
This study aimed at identifying socioeconomic, environmental, and behavioural factors associated with leprosy occurrence.
Low educational level and having experienced food shortage at any time in life were significant socioeconomic risk factors for leprosy.
Individuals who bathed weekly in open water bodies 10 years ago and those with low frequency of changing bed linen recently were more likely to have leprosy.
Previous BCG vaccination was found to be protective (vaccine effectiveness 52%).
We thank Dr Odorico Monteiro and Dra Ivana Cristina de Holanda Cunha Barrêto from the Secretaria de Saúde de Sobral and Faculdade de Medicina da Universidade Federal do Ceará, campus de Sobral for valuable support in data collection. LRSK-P and HF thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—project number 470467/01-0), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Deutscher Akademisher Austauschdienst (DAAD) for supporting the research and the exchange programme between the Fachbereich Humanmedizin of the Freie Universität Berlin and the Universidade Federal doCeará, and CAPES for supporting the PROCAD (Programa Nacional de Cooperação Acadêmica—project 0119/01–6), and exchange programme between the Universidade Federal da Bahia and the Universidade Federal do Ceará.
This research was supported CNPq (project 470467/01-0), by DAAD, and the PROCAD (Programa Nacional de Cooperação Acadêmica)—project 0119/01-6—CAPES.
References
Fine PE, Sterne JA, Ponnighaus JM et al. Household and dwelling contact as risk factors for leprosy in northern Malawi.
van Beers SM, Hatta M, Klatser PR. Patient contact is the major determinant in incident leprosy: implications for future control.
International Leprosy Association Technical Forum. Report of the International Leprosy Association Technical Forum.
Visschedijk J, van de Broek J, Eggens H, Lever P, van Beers S, Klatser P. Review: Mycobacterium leprae—millennium resistant! Leprosy control on the threshold of a new era.
Desikan KV, Sreevatsa. Extended studies on the viability of Mycobacterium leprae outside the human body.
World Health Organization. Leprosy. Global situation.
World Health Organization. Leprosy. Global situation.
FUNASA. Situação da Prevenção e Controle das Doenças Transmissíveis no Brasil. Brasil: Ministério da Saúde,
FUNASA. Boletim Epidemiológico, Edição Especial. Brasil: Ministério da Saúde,
IBGE (Fundação Instituto Brasileiro de Geografia e Estatística), 2000. Dados obtidos on-line, Dezembro de 2003 (http://www.ibge.gov.br/.IBGE) (Fundação Instituto Brasileiro de Geografia e Estatística), 2000. Dados obtidos on-line, Dezembro de
Kerr-Pontes LRS, Montenegro ACD, Barreto ML, Werneck GL, Feldmeier H. Inequality and leprosy in Northeast Brazil: an ecological study.
Ridley DS, Jopling WH. Classification of leprosy according to immunity—five-group system.
Ponnighaus JM, Fine PE, Stern JA, Malema SS, Bliss L, Wilson RJ. Extended scholling and housing conditions are associated with reduced risk of leprosy in rural Malawi.
van Beers SM, de Wit LY, Klaster RP. The epidemiology of Mycobacterium leprae: recent insight.
Sterne JAC, Ponnighaus JM, Fine PEM, Malema SS. Geographic determinants of leprosy in Karonga district, Northen Malawi.
Hansen GA, Looft C. Leprosy: In its Clinical and Pathological Aspects. Bristol: John Wright,
Matsuoka M, Izumi S, Budiawan T, Nakata N, Saeki K. Mycobacterium leprae DNA in daily using water as a possible source of leprosy infection.
Cunha SS, Rodrigues LC, Pedrosa V, Dourado IM, Barreto ML, Pereira SM. Neonatal BCG protection against leprosy: a study in Manaus, Brazilian Amazon.
Meyers WM, Gormus BJ, Walsh GP. Nonhuman sources of leprosy.
Job CK, Harris EB, Allen JL, Hastings RC. Thorns in armadillo ears and noses and their role in the transmission of leprosy.