-
PDF
- Split View
-
Views
-
Cite
Cite
Fabiane M. Santos, Wanderson G. Lima, André S. Gravel, Tassiane A. F. Martins, André Talvani, Rosália M. Torres, Maria Terezinha Bahia, Cardiomyopathy prognosis after benznidazole treatment in chronic canine Chagas' disease, Journal of Antimicrobial Chemotherapy, Volume 67, Issue 8, August 2012, Pages 1987–1995, https://doi.org/10.1093/jac/dks135
Close - Share Icon Share
Abstract
To evaluate the effects of benznidazole on Chagas' disease cardiac prognosis using an experimental dog model of infection.
A total of 28 dogs were divided into three groups: 10 were infected with Trypanosoma cruzi and treated benznidazole during the chronic phase, 10 were infected but untreated, and 8 were non-infected/healthy. The trypanocidal efficacy was measured by parasite kDNA detection in blood and cardiac tissue samples. The effects of benznidazole in ameliorating the cardiac systolic function were evaluated by echodopplercardiogram.
The benznidazole initially induced a potent suppression of parasitaemia in treated animals. However, 12 months post-treatment, the parasite kDNA detections were similar between infected groups. In the baseline echocardiographic parameters there was no variation among all animals. Similarly, 1 month post-treatment there was no significant difference among healthy and infected animals with regard to systolic function. At 12 months post-treatment, an increase in cardiac chamber size related to cardiomegaly was detected among treated and untreated animals, but not in the healthy controls. Interestingly, in spite of both groups of infected animals developing a decrease in their systolic cardiac function, this decline was slightly less in the treated animals. We also evaluated levels of tumour necrosis factor-α and interleukin-10 in peripheral blood mononuclear cell culture supernatant. Cytokine profiles were similar between infected animal groups and correlated with alterations in cardiac function.
The temporary suppression of the T. cruzi infection induced by benznidazole treatment was efficient in reducing systolic cardiac function alterations, but not in preventing the development of cardiomyopathy.
Introduction
A century after its discovery,1 American trypanosomiasis, or Chagas' disease, remains a serious health problem in Latin America, where it affects around 8–10 million people. The acute phase of the disease is associated with high levels of parasitaemia and mild, non-specific symptoms in the majority of infected individuals.2 After 2–4 months, infected individuals pass into the chronic phase, in which parasites are no longer detectable by peripheral blood microscopy. Chronic cardiomyopathy is the most serious manifestation of Chagas' disease and is one of the primary causes of heart failure and other clinical manifestations, including cardiac arrhythmias, heart blockages and arterial or venous thromboembolisms.3 Clinical presentation varies widely according to the degree of myocardial damage, and the signs of the cardiomyopathy evolution can be monitored by electrocardiogram and echocardiography.4,5
Current specific anti-Trypanosoma cruzi chemotherapies are unsatisfactory. These therapies are based on nitroimidazole or nitrofuran agents such as benznidazole (Rochagan®, Rodanil®, Roche) and nifurtimox (Lampit®, Bayer, recently discontinued), which were developed empirically three decades ago.6,7 The major limitation of these compounds is their lower antiparasitic activity in the chronic phase of the disease: 80%–100% of treated patients are not cured.8–11 Several studies have suggested that aetiological treatment in the chronic phase, although it failed to exterminate the parasite, prevented clinical and electrocardiographic changes associated with progression of the disease.10,12–14 However, other studies have conflicted with these findings, concluding that specific treatment failed in preventing the progression of the disease or its complications.9,11,15
The efficacy of specific treatment during the chronic stage of Chagas' disease in preventing the progression of disease evolution is very controversial. Furthermore, studies using experimental models or following chronic chagasic patients are scarce. For these reasons, we investigated the effects of benznidazole treatment on the reduction of tissue parasitism and its correlation with ameliorating cardiac systolic function during the chronic phase of infection by echodopplercardiographic exams performed before infection and after 6 and 18 months of infection, using the dog as an experimental model.
Materials and methods
T. cruzi stock
The Berenice-78 strain (DTU II), isolated by xenodiagnosis in 1978 from what was considered the first clinical case of the disease in Brazil, from a patient with an indeterminate form of the disease, was used in this study. We have previously shown that this strain was susceptible to benznidazole chemotherapy in acute experimental models.15,16
Experimental animals and infection
A total of 28 mongrel dogs (all 4 months old) obtained from the kennel at the Ouro Preto Federal University (UFOP), Minas Gerais State, Brazil were used in this study. All procedures and experimental protocols were conducted in accordance with the guidelines issued by the Brazilian College of Animal Experimentation (COBEA) and approved by the Ethics Committee in Animal Research at UFOP (protocol number 2008/08). The animals were fed with commercial dog food and water ad libitum. Prior to the study, the animals were de-wormed and vaccinated against several infectious diseases. A total of 20 dogs were inoculated by intraperitoneal route with 4.0 × 103 Berenice-78 strain bloodstream trypomastigotes per kg of bodyweight. Eight age-matched uninfected dogs were used as negative controls.
Drug
Benznidazole (N-benzyl-2-nitro-1-imidazolacetamide) was synthesized at Produtos Roche Q.F.S.A., Rio de Janeiro, Brazil.
Treatment scheme and experimental groups
The infected animals were divided into two experimental groups: (i) 10 dogs were treated with benznidazole at 7 mg/kg twice daily for 60 days; and (ii) 10 dogs were maintained as non-treated controls. An additional 8 animals were maintained as a non-infected and untreated control group. These treatment schemes were previously described by Guedes et al.16 In all chronic stage therapeutic schemes, oral treatment was initiated 120 days post-infection.
Assessment of parasite clearance
Parasite load was monitored by blood and heart tissue PCR assays performed before treatment and after the end of benznidazole treatment: until the first and at the 12th month post-treatment.
For the blood PCR assay, 10 mL of blood from each animal was collected, immediately mixed with an equal volume of 6 M guanidine hydrochloride/0.2 M EDTA solution and maintained at room temperature for 2 weeks. Subsequently, these samples were boiled for 15 min before DNA was extracted from 200 μL aliquots taken from each sample.17 For the tissue PCR assay, half of the animals of each experimental group were euthanized 1 month and the other half 12 months post-treatment by injection with thionembutal (Abbott, São Paulo, Brazil) at 0.5 mL/kg of body weight (0.03 g/mL 0.8% saline solution). Heart (right atrium) fragments were obtained from each dog after euthanization. The samples were frozen at −70°C prior to extraction of DNA using a Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA). Blood and tissue DNA amplification was performed in a total volume of 20 μL that contained 0.1% Triton X-100, 10 mM Tris-HCl (pH 9.0), 75 mM KCl, 5 mM MgCl2, 0.2 mM dATP, 0.2 mM dTTP, 0.2 mM dGTP, 0.2 mM dCTP (all obtained from Sigma, St Louis, MO, USA), 1 μL of Taq DNA polymerase (Invitrogen, Grand Island, NY, USA), 20 pmol each of S35 (5′AAATAATGTACGGG(T/G)GAGATGCATGA3′) and S36 (5′GGGTTCGATTGGGGTTGGTGT3′) primers and 2 μL of DNA from each sample.17 The reaction mixture was subjected to 35 cycles of amplification in an automatic thermocycler (Biocycler, BioSan, Riga, Latvia). The temperature profile was as follows: denaturation at 95°C for 1 min (with a longer initial time of 5 min at 95°C in the first amplification cycle), primer annealing at 65°C for 1 min and extension at 72°C for 1 min, with a final incubation at 72°C for 10 min to extend the annealed primers. The PCR products were visualized by 6% PAGE followed by silver staining. All DNA extraction steps and reaction mixtures used for PCR were monitored and compared with positive and negative controls. The PCR analysis was considered negative after three failed DNA extractions of a given sample.
Cytokine peripheral blood mononuclear cell (PBMC) assay
Blood samples were collected from all dogs in the first month and after 12 months of benznidazole treatment. PBMCs were isolated using Ficoll-Hypaque density gradient centrifugation and were washed three times with RPMI (GIBCO, Grand Island, NY, USA). A total of 3.5 × 106 cells were stimulated with 25 μL of parasite antigens (108 trypomastigotes/mL) derived from trypomastigotes in a final volume of 500 μL; the cells were then incubated at 37°C in a 5% CO2 atmosphere for 3 days followed by collection of supernatant. The interleukin 10 (IL-10) and tumour necrosis factor-α (TNF-α) cytokine levels in the collected culture supernatant were quantified by ELISA according to the manufacturer's instructions (R&D Duoset, R&D, Minneapolis, MN, USA). The reaction was detected by adding peroxidase-conjugated streptavidin followed by the addition of a substrate mixture containing hydrogen peroxide and the chromogen ABTS (Sigma).
Echocardiography
All animals were submitted to echodopplercardiographic exams before infection and on the 1st and 12th month post-treatment (i.e. 6 and 18 months post-infection). Animals were injected intraperitoneally with the anaesthetic thiopental sodium (0.03 g/mL 0.8% saline solution) and placed on an isolant electric surface where the anaesthetized dogs were positioned with their limbs perpendicularly orientated to the body. The left ventricle diastolic and systolic diameters were measured at the M-mode echocardiographic exam to determine the left ventricle fractional shortening (LVFS). The left ventricle ejection fraction (LVEF) was determined by the two-dimensional and M-mode echocardiographic measurements of the left ventricle diastolic and systolic volumes. Both the LVFS and the LVEF parameters are well established to the cardiac systolic function evaluation in the Chagas' disease.4,18 In addition, the left atrium volume (LAV) and left ventricle mass index (LVMI) reviewed to the surface body of each animal were measured by the same echodopplercardiographic exam. All the parameters were monitored using a moving Cypress echocardiographic instrument and are well described to the cardiomyopathy evaluation in Chagas' disease.4,18,19 A two-dimensional echo Doppler cardiogram with M-mode measurements was performed on each animal according to the method described by the American Society of Echocardiography.20
Statistical analysis
The mean cytokine production levels and echocardiographic parameters were compared by analysis of variance followed by Tukey's multiple comparison tests. In all cases, differences were considered significant for P < 0.05. Pearson's correlation test was used to correlate the parameters of systolic function related to cardiac chamber size.
Results
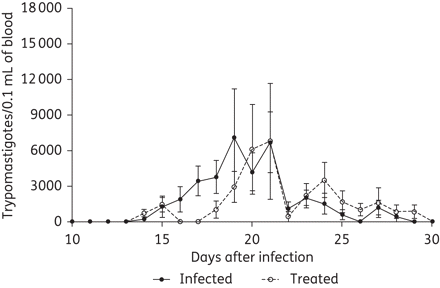
To investigate the effects of benznidazole treatment on the reduction of the parasite load, dogs were treated for 60 days at the chronic stage of disease (i.e. 4 months after infection). The infected animals were divided into two groups: infected treated, which received benznidazole beginning 4 months after infection until the end of 5 months post-infection; and infected non-treated, which was maintained as a control infected group. Parasitaemia in the experimental groups was assessed daily to evaluate the homogeneity of the T. cruzi infections during the acute phase of the infection. Parasites were detected in the peripheral blood of all infected animals; furthermore, the parasitaemia curves were similar in both experimental groups, showing similar parasitaemia levels with peak values of 8182 to 9000 trypomastigotes/0.1 mL of blood and a patent period of 14–15 days (Figure 1).
Parasitaemia curves at the acute stage in mongrel dogs inoculated with 4000 trypomastigotes of the Berenice-78 T. cruzi strain per kg of body weight via an intraperitoneal route. Treated: animals that were later treated with benznidazole during the chronic stage of infection. Infected: animals that remained untreated during the chronic stage of the infection.
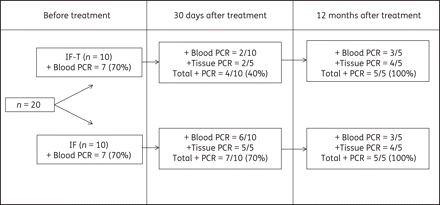
After the application of benznidazole treatment, the parasite load was monitored by PCR analysis of blood and heart tissue performed in the first month post-treatment (at the sixth month of infection) and after 12 months of treatment (at the 18th month of infection). The potent suppression of parasitaemia induced by benznidazole treatment was associated with negative results in the blood PCR analysis performed in the first month after treatment in 80% (8/10) of treated animals compared with 40% (4/10) of infected untreated animals (Figure 2). Similar results were observed in heart tissue PCR analysis in the same period, where parasite kDNA was detected in only 40% (2/5) of the treated animals euthanized 1 month post-treatment. In contrast, 100% (5/5) of the infected non-treated animals euthanized 1 month post-treatment exhibited positive tissue PCR tests. An increase in the frequency of positive results in blood and tissue PCR analysis was detected 12 months post-treatment. Positive results were observed in 60% (3/5) of blood and 80% (4/5) of tissue samples taken from benznidazole-treated animals. For the PCR (blood and tissue PCR assays) performed 12 months post-treatment, parasite kDNA was detected in all of the treated dogs. The same results were detected in blood and tissue PCR tests performed in samples collected from the infected untreated group (Figure 2).
Percentage of positive blood and tissue (right atrium) PCR assay results obtained before treatment as well as 1 month and 12 months post-treatment with 7 mg/kg benznidazole twice daily for 60 days in dogs infected with the Berenice-78 T. cruzi strain. IF: infected untreated animals. IF-T: infected benznidazole-treated animals.
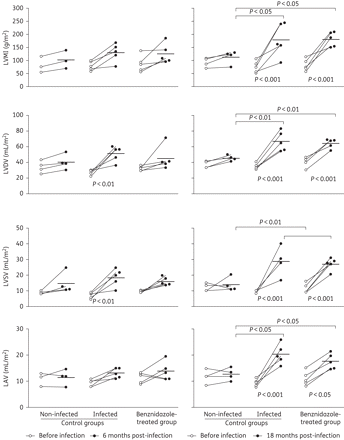
To investigate whether benznidazole treatment in the chronic phase of infection ameliorates cardiac functions, a baseline echocardiogram was performed before the infection and 6 and 18 months after infection (i.e. 1 month and 12 months post-treatment) to evaluate the cardiac function over the period. Figures 3 and 4 show similar baseline characteristics among all experimental groups. The two-dimensional and M-mode echocardiographic analysis performed before infections revealed similar cardiac chamber dimensions and the absence of systolic dysfunction in animals of all experimental groups (Figures 3 and 4). Additionally, there were no variations of the echocardiographic parameters in healthy animals over the evaluated period (i.e. 4–10 or 4–22 months old), i.e. the echocardiographic parameters analysed in this study were not related to the age of the animals.
Evaluation of cardiomegaly by echocardiography performed in mongrel dogs before the infection (white circles) as well as 6 and 18 months post-infection (black circles). The results represent those obtained of each animal from control non-infected, infected untreated and infected benznidazole-treated groups. Difference was considered significant between: (i) the results of the evaluations performed among the animals of each experimental group (below); and (ii) among animals of the different experimental groups (above). P value <0.05 compared by Tukey's non-parametric test. All significant results are shown in the figure.
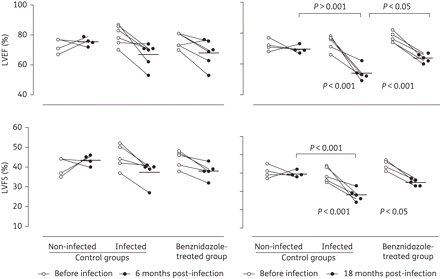
Evaluation of the left ventricle systolic function (LVEF and LVFS) by echocardiography performed on mongrel dogs before the infection (white circles) as well as 6 and 18 months post-infection (black circles). The results shown (mean ± SD) represent those obtained from animals from control non-infected, infected untreated and infected benznidazole-treated groups. *Statistical significance (P < 0.05, as assessed using Tukey's test and the non-parametric test).
Similarly, 6 months after infection (first month post-treatment) there were no significant differences among the healthy and the infected animals, treated or not, with regard to systolic function (Figure 4). However, a significant growth in the left ventricle systolic volume (LVSV) and left ventricle diastolic volume (LVDV) of animals of the infected untreated group (Figure 3) relative to baseline was observed, showing the possibility of later alterations on the LVEF, an important marker of the systolic function as determined by the relation with LVSV and LVDV.
In contrast, significant alterations in echocardiographic parameters related to cardiac chamber growth were detected in all infected animals 18 months post-infection (i.e. 12 months post-treatment). All infected animals exhibited higher values for LVMI, LVDV, LVSV and LAV. The degrees of these alterations were similar among infected benznidazole-treated and infected untreated animals and were significantly higher than those observed in healthy dogs (Figures 3 and 5).
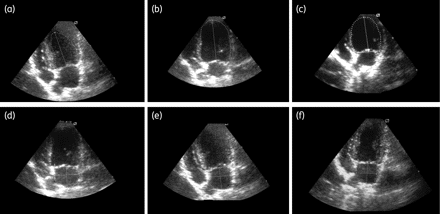
LVDV (a, b and c) and LAV (d, e and f) performed on echodopplercardiographic heart images of mongrel dogs after 18 months of T. cruzi infection (12 months post-treatment with benznidazole). (a and d) Non-infected dog. (b and e) Infected non-treated dog. (c and f) Infected treated dog.
In contrast, benznidazole treatment slightly ameliorated the development of alterations in systolic cardiac function in chronic Chagas' disease. Infected untreated animals exhibited significant reductions in LVEF (54.6 ± 4.9%) and LVFS (28.0 ± 3.4%) compared with healthy dogs (69.5 ± 2.5% and 39.0 ± 2.2% for LVEF and LVFS, respectively). Intermediate values of LVEF (62.6 ± 5.2%) and LVFS (33.6 ± 3.4%) were observed in benznidazole-treated animals; additionally, the differences between the healthy and benznidazole-treated infected dogs were not significant (Figure 4). However, both groups infected with T. cruzi (treated or not) experienced a significant decrease in cardiac systolic function at the 18th month of infection compared with baseline, although the decline was slightly less for the benznidazole-treated dogs (Figure 4).
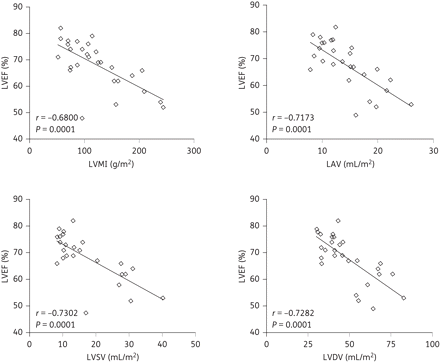
We observed that the reduction of the LVEF was clearly associated with the growth of the cardiac chamber dimensions (LVMI, LVDV, LVSV and LAV) probably related to the heart inflammatory process (Figure 6).
Pearson's correlation among the LVEF and LVMI, LAV, LVSV and LVDV.
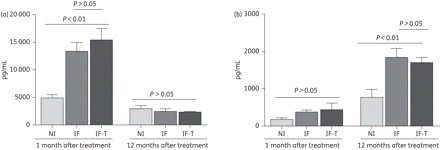
We also evaluated the levels of TNF-α and IL-10 in the supernatants of PBMC cultures of infected and healthy dogs to correlate cardiac alterations with cytokine levels. The cytokine profile was similar among infected animals and correlated with alterations in cardiac function. Antigenic stimulation of PBMCs from infected animals 6 months after inoculation (i.e. until the first month post-treatment) led to a significant enhancement in the production of IL-10 compared with the healthy control group (Figure 7a). However, 18 months post-inoculation (i.e. 12 months post-treatment) there was a reduction in the level of IL-10 (Figure 7a) and an increase in TNF-α production (Figure 7b). Taken together, these observations suggest that TNF-α mediated the formation of chronic myocardial lesions and it is possibly related to the functional alterations observed in cases of chronic Chagas' disease cardiomyopathy.
Quantification of IL-10 (a) and TNF-α (b) levels by ELISA (6 and 18 months post-infection) in PBMC culture supernatants of mongrel dogs. The results (mean ± SD) represent those obtained from animals from control non-infected (NI), infected untreated (IF) and infected benznidazole-treated (IF-T) groups. Statistical significance P < 0.05 (as assessed using Tukey's test and the non-parametric test).
Discussion
Cardiac involvement in Chagas' disease is the major mechanism responsible for the high morbidity and mortality. Given the lack of therapeutic options for Chagas' disease, the potential benefits of benznidazole treatment during the chronic phase aiming at cardiac function preservation should be carefully investigated. In the present study we used a canine model to verify the ability of benznidazole to induce a decrease in parasitic load and, later, under the evolution of cardiomyopathy and heart chronic systolic dysfunction.
Several studies using mice treated with benznidazole during chronic T. cruzi infections have demonstrated that this drug did not completely eliminate the parasite, but induced a reduction in the parasite load.13 Similarly, studies that investigated the effect of benznidazole treatment in cases of human chronic T. cruzi infection demonstrated a reduction in the parasite load in blood culture and blood PCR tests.21,22
In this study, negative blood PCR results were observed in 80% (8/10) of the benznidazole-treated animals 30 days post-treatment during the chronic phase of the infection. As PCR is a well-established, sensitive and specific tool for assessing the susceptibility of the parasites to specific treatments, our results demonstrate the effectiveness of benznidazole in clearing parasites over a short period. However, an increase in parasitaemia was detected 12 months after treatment: only 40% (2/5) of the treated animals exhibited negative blood PCR test results. Using blood PCR as a cure control, treatment effectiveness levels of 37.5%–60.4% have been reported in patients and experimental models treated with benznidazole during the chronic phase of Chagas' disease.16,22 Interestingly, when heart-tissue PCR assays were performed, positive results were detected in 40% (2/5) and 80% (4/5) of treated animals in tissue samples evaluated 1 month and 12 months post-treatment, respectively. These data confirm the immediate suppression of parasitism resulting from treatment with benznidazole and the rebounding in long-term parasitism after treatment.
Other studies have reported important differences using parasitological methods (i.e. blood culture and xenodiagnosis methods) or blood/tissue PCR performed after administration of benznidazole treatment to humans or experimental models with chronic infection including: (i) 60%–88% frequency of negative results using blood culture or xenodiagnostic tests;16,21,23 (ii) 0%–62.5% negative T. cruzi kDNA detection results by blood PCR;11,16,22 and (iii) ∼30% negative results using heart-tissue PCR.24 However, our study revealed that such observed negative results are not stable; specifically, an increase in positive test results was observed in both heart-tissue and blood PCR tests after 12 months of benznidazole treatment.
Recent studies using sensitive detection methods, such as PCR assays, have demonstrated clear correlations between the presence of the parasite and the pathological processes associated with chronic Chagas' disease.2,25–27 In this study, the characteristic cardiac dysfunctions commonly observed during the chronic stage of infection were measured at the same time that parasite detection tests were performed. Echocardiographic parameters for cardiomegaly and systolic dysfunction were evaluated in a dog model both before treatment as well as 1 month and 12 months post-treatment.
The benznidazole treatment did not prevent echocardiographic alterations related to cardiomegaly. All analysed parameters, including LAV, revealed that the increase in cardiac chamber size was similar between infected treated and untreated animals and was significantly higher than that observed in healthy animals. In addition, an increase in LAV has been shown to have great prognostic value in patients with dilated cardiomyopathy.19 The assessment of this parameter is important for the management of chagasic patients with no apparent heart disease and, furthermore, LAV can be useful to monitor the possible occurrence of mitral regurgitation or LVEF reduction.19,28,29
Interestingly, treatment with benznidazole slightly ameliorated alterations in systolic cardiac functions; for example, the parameters LVEF and LVFS were similar among infected treated and non-infected control animals. Infected animals that did not receive benznidazole treatment exhibited lower values for these systolic cardiac parameters at 18 months post-infection. However, when LVEF and LVFS values of all experimental animals were analysed with regard to their baseline data, both the benznidazole-treated and untreated T. cruzi-infected groups revealed systolic cardiac function deterioration 18 months after inoculation, which was slightly less for the benznidazole-treated dogs. Healthy control animals exhibited similar LVEF and LVFS values along all the evaluated period. Left ventricular systolic dysfunction has been described as an important feature of cardiomyopathy prognosis and cardiac risk-factor evolution.5,18,30 The association of LVEF reduction with a growth in left ventricle volumes (LVSV and LVDV) and parasympathetic dysautonomia are considered to be important predictors of mortality.4,31 In the present work significant correlations were observed between LVEF reduction and the growth of the left ventricle volumes (LVSV and LVDV), LVMI and LAV.
According to recent studies, the presence of the parasite and the inflammatory process underlie the pathological evolution of chronic Chagas' disease.26,32 In this work, cytokine synthesis by PBMCs was measured at the same time that parasite detection tests and cardiac function evaluation were performed. Interestingly, a change in the IL-10 and TNF-α production levels was observed in infected dogs. Immediately after benznidazole treatment (i.e. 6 months after infection), the regulatory cytokine IL-10 was expressed at higher levels than the inflammatory cytokine TNF-α in both the groups of infected animals in comparison with healthy dogs. Twelvemonths post-treatment, the expression patterns of these cytokines were inverted, with higher TNF-α levels and lower IL-10 levels being expressed by PBMCs isolated from infected animals (both treated and non-treated). Other studies have demonstrated that the development of cardiomyopathy in Chagas' disease correlated with the production of high levels of inflammatory cytokines (TNF-α and interferon-γ) and low levels of regulatory cytokines (IL-10 and interleukin 4).33–35 Some authors demonstrated that elevated levels of TNF-α, previously associated with the development of cardiac lesions,36–38 represent a marker of left ventricular dysfunction in patients with Chagas' disease cardiomyopathy. The data presented here demonstrating an increase in TNF-α levels and ventricular dysfunction in a dog model match the immune responses and cardiac alterations described in cases of human T. cruzi infection. The elevation of TNF-α synthesis and the reduction in IL-10 synthesis by PBMCs were not altered by benznidazole treatment: these results were verified in both groups of infected animals (i.e. both treated and non-treated).
An overall analysis of our results indicates that delivery of benznidazole treatment during the chronic stage of Chagas' disease in mongrel dogs infected with the T. cruzi Berenice-78 strain was not able to efficiently prevent left ventricular long-term systolic cardiac dysfunction; and, it did not prevent the increase in cardiac chamber size that may be a result of the immunological imbalance of TNF-α and IL-10 synthesis by PBMCs, with high levels of TNF-α and low levels of IL-10 contributing to the progression of cardiomyopathy disease. Furthermore, other analysis of cardiac function including the evaluation of the diastolic and electric alterations as well as the study of the inflammatory process in heart muscle tissue could be important to better clarify the impact of benznidazole treatment on the progression of cardiomyopathy disease.
Funding
This work received financial support from the Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (Cnpq) and Universidade Federal de Ouro Preto, and research fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (M. T. B.).
Transparency declarations
None to declare.
Author contributions
All authors contributed to the conception and design of the study, laboratory analysis, data analysis and interpretation, and drafting of the manuscript. All authors contributed to and read and approved the manuscript. M. T. B. is guarantor of the paper.
Acknowledgements
We gratefully thank Geovan P. Crepalde for assistance with the mongrel dogs.