-
PDF
- Split View
-
Views
-
Cite
Cite
Patrick B. Kyle, Kathryn B. Brown, Alyssa P. Bailey, John L. Stevenson, Reactivity of Commercial Benzodiazepine Immunoassays to Phenazepam, Journal of Analytical Toxicology, Volume 36, Issue 3, April 2012, Pages 207–209, https://doi.org/10.1093/jat/bks008
Close - Share Icon Share
Abstract
Phenazepam is a long acting benzodiazepine that is not controlled in Canada, the United States or many European countries. The abuse of phenazepam has gained recent attention due to the number of hospitalizations and fatalities resulting from overdose. The compound is relatively potent with recommended doses of 0.5–1.0 mg, or 1/10th the recommended dose of diazepam, and is easy to obtain locally or from international suppliers via the internet. Increased risk of phenazepam overdose is attributed to its potency and the forms in which it is supplied. Individuals without scales or balances are prone to estimate dose amounts of powder visually, which can result in significant errors. The detection of phenazepam has been described using various methods including GC, GC/MS and LC/MS, but no data regarding the sensitivity of commercially available immunoassays exist. In this study, phenazepam-spiked urine samples were analyzed using five commercial instruments and two point of care devices. The concentrations of phenazepam required for detection ranged from 140–462 ng/mL, which is comparable to those of other benzodiazepines. Laboratorians and clinicians should be confident that phenazepam will be detected via most commercial drugs of abuse screens in patients after significant ingestion.
Introduction
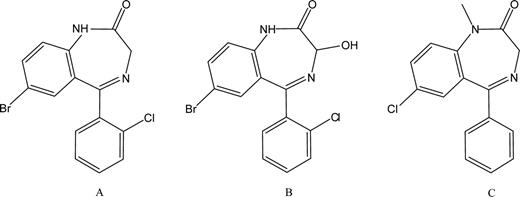
Phenazepam [7-bromo-5-(-2-chlorophenyl)-1,3-dihydro-1,4-benzodiazepin-2-one] was developed in the Soviet Union and has been used since the 1970s as an anxiolytic, anticonvulsant and sedative-hypnotic in Russia and several Commonwealth of Independent States (1). The compound shares structural similarity with other commonly prescribed benzodiazepines, including diazepam and lorazepam (Figure 1). Like other benzodiazepines, its mechanism of action involves modulation of GABA(A) receptors (2). Phenazepam exhibits a half-life of approximately 60 h in humans (3), and is metabolized to the active metabolite 3-hydroxyphenazepam (Figure 1).
Molecular structures of phenazepam (A), 3-hydroxyphenazepam (B) and diazepam (C).
Phenazepam is not controlled in Canada, the United Kingdom, the United States or many European countries. As a result, phenazepam is relatively easy to obtain. Tablets may be found at local head shops, and many international suppliers offer the drug via secure and confidential internet transactions. Phenazepam has recently gained much attention due to the number of hospitalizations resulting from its abuse (4, 5, 6). Its presence has been noted in several US states, including Kentucky, Mississippi, New York, North Carolina and Wisconsin (7, 8, 9).
Increased risk of overdose is associated with illicit use of phenazepam due to its potency and the forms in which it is supplied. The recommended dose is 0.5–1.0 mg, or 1/10th the dose of diazepam. Potential for toxicity also exists because phenazepam is commonly obtained in 100–1,000 mg quantities as bulk powder via the internet. Individuals without access to analytical balances often estimate dose amounts visually instead of by mass, which often results in significant errors. This combination of accessibility and potency makes the drug increasingly popular and dangerous. Due to its potency and long half-life, many individuals experience extended sedation and/or amnesia after ingesting sufficient quantities. One report from the Swedish Poisons Information Centre assessing the acute toxicity of phenazepam found that 14 of 61 (23%) of hospitalized patients experienced symptoms five days post-ingestion (10).
The safety profile of benzodiazepines is more favorable than barbiturates, but large doses can cause respiratory depression and coma (11). Death from ingestion of benzodiazepines alone is rare, except when combined with other central nervous system depressants that act synergistically (12, 13). Recent reports of fatal phenazepam overdose in the United States include an 18-year-old male who died after ingesting phenazepam and oxycodone (14), and a 42-year-old male who died after ingesting phenazepam and poppy seed tea, which resulted in combined toxicity from phenazepam, morphine, codeine and thebaine (15).
Detection of phenazepam has been described using various means, including gas chromatography with electron capture (16) or mass spectrometry detection (17), and liquid chromatography–mass spectrometry (15). However, the detection of phenazepam using commercially available immunoassays has not been reported, and no statement regarding its cross-reactivity could be found in the technical literature of several manufacturers' benzodiazepine assays. In this study, we determine the urine concentrations of phenazepam required for its detection via five commercial instruments and two point-of-care devices.
Methods
Phenazepam (purity >99%) was obtained from Lipomed (Cambridge, MA). Optima-grade methanol was obtained from Fisher Scientific (Pittsburgh, PA). Human urine was obtained within the laboratory and was confirmed free of drugs using gas chromatography–mass spectrometry, as previously described (18). A stock solution of phenazepam (1 mg/mL) was created by dissolving the drug in methanol. Standard solutions of phenazepam (100–10,000 ng/mL) were created by adding appropriate aliquots of the stock solution to drug-free urine. The following instruments, assays, and point of care devices were used during the study: Standard solutions of increasing phenazepam concentrations were analyzed per manufacturers' protocols using each of the methods listed previously. Upon obtaining a positive result, standard solutions in a narrow concentration range were re-analyzed until a minimum positive concentration was identified. All analyses were performed in triplicate to account for assay imprecision and to ensure accuracy.
Roche Cobas 6000 c501 (Roche Diagnostics, Indianapolis, IN) using the Roche Cobas Benzodiazepines Plus (BENZ) assay;
Siemens V-twin (Siemens Healthcare Diagnostics, Deerfield, IL) using the Siemens Syva Emit II Plus Benzodiazepine assay;
VITROS 5,1FS (Ortho-Clinical Diagnostics, Rochester, NY) using the VITROS BENZ Benzodiazepines assay;
Beckman Coulter AU400 (Beckman Coulter, Brea, CA) using the Beckman Coulter Emit II plus Benzodiazepine assay;
TECAN Freedom EVO 75 (Tecan US, Durham, NC) using the IMMUNALYSIS Benzodiazepines ELISA assay (Immunalysis, Pomona, CA);
ATN FasTox 12 CLIA-Waived Multiple Drug Dipcard (Advanced Toxicology Network, Memphis, TN); and
Bio-Rad TOX/See Drug Screen Test (Bio-Rad Laboratories, Hercules, CA).
Results
Phenazepam was detected by each of the methods tested (Table I) in concentrations comparable to other similar benzodiazepines (19). The drug was detected in lower than designated cutoff concentrations in five of the seven methods used.
Concentrations of Phenazepam Equivalent to Positive Cutoffs of Seven Commercial Benzodiazepine Immunoassays
| Instrument . | Assay . | Positive cutoff (analyte) . | Phenazepam equivalent . | Cross-reactivity . |
|---|---|---|---|---|
| Siemens V-Twin | Siemens Emit II Plus | 200 ng/mL (Lormetazepam) | 142 ng/mL | 141% |
| Roche c 501 | Roche Cobas Benz Plus | 300 ng/mL (Nordiazepam) | 362 ng/mL | 83% |
| Vitros 5,1 FS | Vitros BENZ | 200 ng/mL (Lormetazepam) | 168 ng/mL | 119% |
| Olympus AU400 | Siemens Emit II plus | 200 ng/mL (Lormetazepam) | 140 ng/mL | 143% |
| Tecan Freedom Evo | Immunalysis Benzo ELISA | 200 ng/mL (Oxazepam) | 462 ng/mL | 43% |
| POC Device | ||||
| ATN FasTox | ATN FasTox | 300 ng/mL (Oxazepam) | 170 ng/mL | 176% |
| Bio-Rad Tox/See | BioRad Tox/See | 300 ng/mL (Oxazepam) | 212 ng/mL | 142% |
| Instrument . | Assay . | Positive cutoff (analyte) . | Phenazepam equivalent . | Cross-reactivity . |
|---|---|---|---|---|
| Siemens V-Twin | Siemens Emit II Plus | 200 ng/mL (Lormetazepam) | 142 ng/mL | 141% |
| Roche c 501 | Roche Cobas Benz Plus | 300 ng/mL (Nordiazepam) | 362 ng/mL | 83% |
| Vitros 5,1 FS | Vitros BENZ | 200 ng/mL (Lormetazepam) | 168 ng/mL | 119% |
| Olympus AU400 | Siemens Emit II plus | 200 ng/mL (Lormetazepam) | 140 ng/mL | 143% |
| Tecan Freedom Evo | Immunalysis Benzo ELISA | 200 ng/mL (Oxazepam) | 462 ng/mL | 43% |
| POC Device | ||||
| ATN FasTox | ATN FasTox | 300 ng/mL (Oxazepam) | 170 ng/mL | 176% |
| Bio-Rad Tox/See | BioRad Tox/See | 300 ng/mL (Oxazepam) | 212 ng/mL | 142% |
Concentrations of Phenazepam Equivalent to Positive Cutoffs of Seven Commercial Benzodiazepine Immunoassays
| Instrument . | Assay . | Positive cutoff (analyte) . | Phenazepam equivalent . | Cross-reactivity . |
|---|---|---|---|---|
| Siemens V-Twin | Siemens Emit II Plus | 200 ng/mL (Lormetazepam) | 142 ng/mL | 141% |
| Roche c 501 | Roche Cobas Benz Plus | 300 ng/mL (Nordiazepam) | 362 ng/mL | 83% |
| Vitros 5,1 FS | Vitros BENZ | 200 ng/mL (Lormetazepam) | 168 ng/mL | 119% |
| Olympus AU400 | Siemens Emit II plus | 200 ng/mL (Lormetazepam) | 140 ng/mL | 143% |
| Tecan Freedom Evo | Immunalysis Benzo ELISA | 200 ng/mL (Oxazepam) | 462 ng/mL | 43% |
| POC Device | ||||
| ATN FasTox | ATN FasTox | 300 ng/mL (Oxazepam) | 170 ng/mL | 176% |
| Bio-Rad Tox/See | BioRad Tox/See | 300 ng/mL (Oxazepam) | 212 ng/mL | 142% |
| Instrument . | Assay . | Positive cutoff (analyte) . | Phenazepam equivalent . | Cross-reactivity . |
|---|---|---|---|---|
| Siemens V-Twin | Siemens Emit II Plus | 200 ng/mL (Lormetazepam) | 142 ng/mL | 141% |
| Roche c 501 | Roche Cobas Benz Plus | 300 ng/mL (Nordiazepam) | 362 ng/mL | 83% |
| Vitros 5,1 FS | Vitros BENZ | 200 ng/mL (Lormetazepam) | 168 ng/mL | 119% |
| Olympus AU400 | Siemens Emit II plus | 200 ng/mL (Lormetazepam) | 140 ng/mL | 143% |
| Tecan Freedom Evo | Immunalysis Benzo ELISA | 200 ng/mL (Oxazepam) | 462 ng/mL | 43% |
| POC Device | ||||
| ATN FasTox | ATN FasTox | 300 ng/mL (Oxazepam) | 170 ng/mL | 176% |
| Bio-Rad Tox/See | BioRad Tox/See | 300 ng/mL (Oxazepam) | 212 ng/mL | 142% |
Discussion
One limitation of this study is that the detection of metabolite 3-hydroxyphenazepam was not investigated. Accumulation of 3-hydroxyphenazepam in the blood is negligible (3), but urinary concentrations have not been reported. This metabolite is analogous to temazepam, the 3-hydroxy metabolite of diazepam, and its detection via immunoassay may be comparable.
This study reveals that the sensitivities of commercial immunoassays to phenazepam are comparable to their sensitivities toward other benzodiazepines (20). In most instances, phenazepam was detected in concentrations equivalent to or less than the assays' reference cutoff concentrations. Phenazepam was detected in clinically relevant concentrations with every method tested. Therefore, laboratorians should expect positive benzodiazepine immunoassay results in patient samples after significant phenazepam ingestion.
Clinicians should be aware of the widespread availability and increasing illicit use of phenazepam. Phenazepam use is on the rise because it is easy to obtain and less expensive than other benzodiazepines. The drug is increasingly being ingested by habitual drug users in combinations with other drugs, which may either mask or potentiate its effects. The benzodiazepine antagonist flumazenil is reported to be efficacious in overdose, but multiple doses may be required due to the long half-life of phenazepam. As with other long-acting benzodiazepines, care should focus on ensuring a patent airway and supportive measures, and evaluating for co-ingested substances.
Due to current classification schemes, US law enforcement agencies can only report phenazepam as a “non-narcotic substance.” Agencies may not even report phenazepam as a controlled substance “analog” because benzodiazepines are not classified as Schedule I or II. Therefore, prosecution of sales or possession is difficult, and abuse will increase. We will continue to observe the clinical sequela from phenazepam abuse until major restrictions are placed on this compound.