-
PDF
- Split View
-
Views
-
Cite
Cite
Krishna Mohan Vadrevu, Venugopal Potula, Vasant Khalatkar, Niranjana S Mahantshetty, Atish Shah, Raches Ella, Persistence of Immune Responses With an Inactivated Japanese Encephalitis Single-Dose Vaccine, JENVAC and Interchangeability With a Live-Attenuated Vaccine, The Journal of Infectious Diseases, Volume 222, Issue 9, 1 November 2020, Pages 1478–1487, https://doi.org/10.1093/infdis/jiz672
Close - Share Icon Share
Abstract
This study reports immunogenicity, safety, and interchangeability of a single-dose, inactivated, Vero-cell derived, JENVAC to the live-attenuated SA 14-14-2 vaccine in healthy children.
This phase 4, multicenter, open-label, randomized, control trial enrolled 360 children who were equally randomized to receive a single dose of either JENVAC or SA 14-14-2. Children were followed at various time points, until 2 years (day 720) postvaccination, upon which a subset from each group was divided and allocated to a receive a booster dose or the other vaccine.
At all time points, immunological measures were statistically higher in the JENVAC group. In the interchangeability study, children receiving 2 doses of JENVAC reported significantly higher response compared with 2 doses of SA 14-14-2. No difference in adverse events was observed. These corroborate with excellent seroprotection after the first dose of an earlier JENVAC study.
A single-dose vaccination with JENVAC induces protective titers that persist up to 1 year. We report appreciable interchangeability between both vaccines, with JENVAC/JENVAC combination exhibiting the highest immune response. JENVAC is now licensed as a single-dose Japanese encephalitis vaccine.
Japanese encephalitis (JE) virus is a leading cause of acute encephalitis in Asia [1]. Children below the age of 14 years and travelers who visit endemic areas are at the highest risk of acquiring this infection [1, 2]. The World Health Organization (WHO) recommends the use of JE vaccine in national immunization programs (NIPs) in all areas where JE is recognized as a public health priority [3]. Currently, there are 15 JE vaccines in use globally. With relevance to this study, only a few vaccines are mentioned. The live-attenuated lyophilized vaccine, CD.JEVAX, is notably the most widely administered JE vaccine. It was derived from the wild-type SA 14-14-2 strain in Primary Hamster Kidney Cells and is manufactured by Chengdu Biologicals, China [4]. New-generation, Vero cell-derived inactivated vaccines are gaining favor with regards to conferring longer lasting immunity. IXIARO is an inactivated cell-derived vaccine, based on SA 14-14-2 strain, manufactured by Valneva, Scotland [5, 6], and the same vaccine is produced in India as JEEV (Biological E. Ltd) [7].
JENVAC, manufactured by Bharat Biotech, India was initially licensed as a 2-dose vaccine in 2014 [8]. This inactivated Vero cell-adapted vaccine is produced based on the Kolar strain 821564XY, which was isolated from the peripheral blood of a viremic individual during a JE outbreak in India. The strain was identified based on neutralization indexes to JE (Nakayama) strain [9]. Most JE vaccines are recommended as 2-dose regimens (primary immunization series) often requiring an additional booster dose at a later time point [10, 11].
In contrast to excellent efficacy results of the widely used SA 14-14-2 in China, South Korea, and Nepal [12–15], the efficacy and immunogenicity in India were found to be lower postvaccination [8, 16, 17], and the second dose of this vaccine has been recommended. Hence, it is essential to determine the long-term immune response to a single-dose regimen. Such a regimen would also be beneficial for mass vaccination programs to improve vaccine coverage [18].
We previously reported that a 2-dose regimen (administered 4 weeks apart) of JENVAC was well tolerated and immunogenic in a phase 2/3 study of age groups from 1 to 50 years. In this study, seroprotection (SPR) rates for SA 14-14-2 (postsingle dose) were 79.6% after 28 days, whereas the corresponding value for JENVAC (postsingle dose) was 98.7%. JENVAC (2-dose) demonstrated a significantly higher immune response than the SA 14-14-2 vaccine at all other time points [8]. Significant effort was made to contact and collect serum from the participants who dropped out (received 1 dose of either vaccine). Immune responses for JENVAC were higher at 1 and 2 years, postvaccination, albeit with low sample size. The persistence of antibody levels with a single dose of JENVAC in large cohort studies is unknown. Furthermore, since the introduction of Vero cell-derived JE vaccines, there are no published data to support whether they are interchangeable with SA 14-14-2. Based on our previously reported findings and because both vaccines are widely used in India, we conducted a phase 4, open-label, randomized, controlled trial to evaluate and compare (1) single-dose, immunogenicity, and safety of JENVAC and SA 14-14-2 vaccine and (2) interchangeability of a 2-dose regimen of either vaccine.
METHODS
Study Objectives/Endpoints
This study was conducted to compare the long-term immune response and safety profile of a single dose of JENVAC and SA 14-14-2. After successful completion of the single-dose study, children either received a booster of the same vaccine (previously administered) or interchanged to receive the other vaccine (from now on referred to as the interchangeability study). The focus of this study was on subjects in the age group of 1 to 15 years considered as the “vulnerable” age group from a JE disease perspective [1].
Clinical Trial Design and Children
This phase 4 study was a multicenter, randomized, single-blind, clinical trial conducted from June 2014 for the single-dose study and May 18 to July 14, 2016 for the interchangeability study at 4 sites in India. The trial was approved by the Drug Controller General and respective ethical committees. A data safety monitoring board reviewed safety data periodically. The trial was registered with Clinical Trials Registry of India (www.ctri.nic.in; Clinical Trials Registration CTRI/2014/02/004386 and CTRI/2016/05/006909).
Study Children and Trial Design
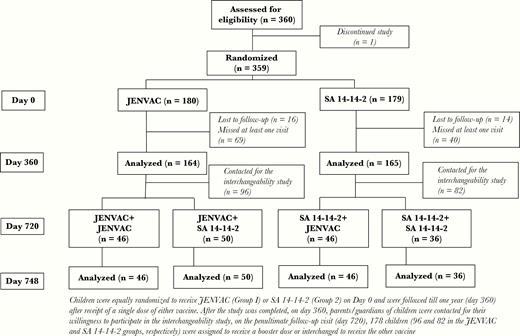
Children were screened for eligibility and enrolled (n = 360) after obtaining written informed consent from the parents and/or legally acceptable representative under audiovisual recording. Children were equally randomized to receive JENVAC (Group 1) or SA 14-14-2 (Group 2) on day 0 and were followed until 1 year after receipt of a single dose of either vaccine. Each group was stratified into 3 age-wise subgroups: (1) ≥1 to ≤5 years, (2) 5> to ≤10 years, and (3) >10 to ≤15 years. After this study was completed, on day 720, parents and/or guardians of children from the parent study were contacted for their willingness to participate in the interchangeability study. Screened children (n = 178) were consented again, enrolled, and assigned (nonrandom) to receive a booster of the same vaccine (previously received) or interchanged to receive the other vaccine. This led to 4 treatment arms: (1) JENVAC + JENVAC, (2) JENVAC + SA 14-14-2, (3) SA 14-14-2 + JENVAC, and (4) SA 14-14-2 + SA 14-14-2 groups, respectively.
Vaccine
Study vaccine was administered as a single dose of JENVAC (5 µg/0.5 mL/dose) administered intramuscularly. The study vaccine was based on the Kolar-821564XY strain. The comparator vaccine was a commercially obtained lyophilized live-attenuated vaccine SA 14-14-2 (≥105.4 plaque-forming units/dose) manufactured by Chengdu Institute of Biological Products, which was administered subcutaneously as a single dose.
Randomization and Blinding
Children were equally randomized to receive JENVAC (Group 1) or SA 14-14-2 (Group 2). Study staff at all sites were unaware of the randomization sequence. Due to the difference in vaccine formulations, JENVAC (liquid), and SA 14-14-2 (lyophilized), field staff were not blinded. A copy of the randomization list of healthy infant identification numbers and decoded key were sent to the biostatistician at the end of the study. Samples collected for serological testing were deidentified. The sponsor and laboratory staff (performing serologic assays) remained blinded to all treatment assignments. Randomization procedures were conducted by a third-party Contract Research Organization, Croissance Clinical Research
Procedures
For the single-dose study, children were screened for eligibility and enrolled after obtaining written informed consent from the parents and/or legally acceptable representative. Children were equally randomized to receive study or comparator vaccine on day 0. Peripheral venous blood samples (2 mL) were obtained on day 0 (before vaccine administration) and on days 28, 56, 90, 180, 360 (postvaccination). For the interchangeability study, samples were collected on day 720 (prebooster) and on day 748 (postbooster/interchange). Clinical data management and analyses were contracted to Sensaas India Pvt. Ltd.
Immunogenicity
Serum was obtained from all the children at all specified time points. Samples were subjected to the determination of 50% plaque reduction neutralization titer (PRNT50). In brief, heat-inactivated serum was serially diluted 4-fold starting at a dilution of 1:10, incubated with JEV 821564XY, and inoculated onto Vero cells in 6-well plates. The monolayers were overlaid with .84% methylcellulose and incubated for 5 days before staining with 0.1% crystal violet and counting plaques. The PRNT50 was computed by using the formula [X − 50/47.7662] + Y, where X is the percentage of plaques reduced in the next dilution above 50%, and Y is the next log dilution above 50%, as described previously [19]. Serological assays were performed in a blinded manner at Bharat Biotech, after detailed validations in a blinded manner in an external laboratory [8].
The PRNT50 values were transformed to SPR, seroconversion (SCR), and geometric mean titers (GMTs). A PRNT50 of ≥10 U/mL was considered to be seroprotective [20]. Seroconversion was defined as a titer of ≥10 U/mL if the baseline titer (before vaccination on day 0) was <10 U/mL or as a 4-fold increase, if the baseline titer was ≥10 U/mL. The GMTs were calculated as the antilog of the mean of the log transformation of titers.
Reactogenicity and Safety
All children were followed up for safety after vaccination by documentation of adverse events (AEs) between the administration of the first dose and 28 days after the last dose. Children were observed for 30 minutes postvaccination for assessing immediate adverse reactions. Vaccine reactogenicity was documented as events reported within 7 days after vaccination. Detailed safety information for solicited AEs, unsolicited AEs, and serious AEs was collected via subject diary card, telephonic contact by the clinical trial team, and review at each study visit. Serious AEs were reviewed by a Data Safety Monitoring Board.
Statistical Analyses
A sample size of 360 children was calculated assuming 80% power, a noninferiority margin of 10%, and a 95% confidence interval (CI). Assuming a response rate of 90% from the test group and a 75% from the reference group at day 28, children were randomized 1:1 to receive either vaccine assigned by a computer-generated plan with stratification by age.
The per-protocol population was used for the main immunogenicity analysis. Categorical variables were presented as counts and percentages with 95% CIs. Comparison of SPR, SCR, and AEs between groups was performed using χ 2 test with Yate’s correction or Fisher’s exact test. Differences in GMTs was performed using the 2-sample paired t test. An independent organization, Sensaas India Pvt. Ltd, conducted the analyses using SAS version 9.3.
RESULTS
Single-Dose Study
Among 360 children randomized, 329 (91.4%) completed the trial as per protocol, 31 of whom children dropped out either due to loss to follow-up, migration from the trial area, or withdrawal of consent (Figure 1). There were no significant demographic and baseline differences between groups (Table 1).
Demographic Data for the Trial Cohort in Each Treatment Groupa
| Variable . | JENVAC (n = 180) . | SA 14-14-2 (n = 179) . | P value . |
|---|---|---|---|
| Age (years), mean (SD) | 7.4 (3.8) | 7.4 (3.8) | .92 |
| Height (cm), mean (SD) | 113 (22.9) | 113 (21.4) | .99 |
| Weight (kg), mean (SD) | 21.2 (9.4) | 21.2 (9.9) | .99 |
| Females, n (%) | 89 (49.4) | 75 (41.7) | .18 |
| Before vaccination seroprevalence, n (%) | 37 (20.6) | 41 (22.9) | .59 |
| Variable . | JENVAC (n = 180) . | SA 14-14-2 (n = 179) . | P value . |
|---|---|---|---|
| Age (years), mean (SD) | 7.4 (3.8) | 7.4 (3.8) | .92 |
| Height (cm), mean (SD) | 113 (22.9) | 113 (21.4) | .99 |
| Weight (kg), mean (SD) | 21.2 (9.4) | 21.2 (9.9) | .99 |
| Females, n (%) | 89 (49.4) | 75 (41.7) | .18 |
| Before vaccination seroprevalence, n (%) | 37 (20.6) | 41 (22.9) | .59 |
Abbreviations: N, number of children allotment in treatment; SD, standard deviation.
aP values calculated by unpaired t test and χ 2 test. Before vaccination, seroprevalence was defined as the proportion of children with a 50% plaque reduction neutralization titer ≥10 U/mL on day 0.
Demographic Data for the Trial Cohort in Each Treatment Groupa
| Variable . | JENVAC (n = 180) . | SA 14-14-2 (n = 179) . | P value . |
|---|---|---|---|
| Age (years), mean (SD) | 7.4 (3.8) | 7.4 (3.8) | .92 |
| Height (cm), mean (SD) | 113 (22.9) | 113 (21.4) | .99 |
| Weight (kg), mean (SD) | 21.2 (9.4) | 21.2 (9.9) | .99 |
| Females, n (%) | 89 (49.4) | 75 (41.7) | .18 |
| Before vaccination seroprevalence, n (%) | 37 (20.6) | 41 (22.9) | .59 |
| Variable . | JENVAC (n = 180) . | SA 14-14-2 (n = 179) . | P value . |
|---|---|---|---|
| Age (years), mean (SD) | 7.4 (3.8) | 7.4 (3.8) | .92 |
| Height (cm), mean (SD) | 113 (22.9) | 113 (21.4) | .99 |
| Weight (kg), mean (SD) | 21.2 (9.4) | 21.2 (9.9) | .99 |
| Females, n (%) | 89 (49.4) | 75 (41.7) | .18 |
| Before vaccination seroprevalence, n (%) | 37 (20.6) | 41 (22.9) | .59 |
Abbreviations: N, number of children allotment in treatment; SD, standard deviation.
aP values calculated by unpaired t test and χ 2 test. Before vaccination, seroprevalence was defined as the proportion of children with a 50% plaque reduction neutralization titer ≥10 U/mL on day 0.
Immunogenicity
Single-Dose Study
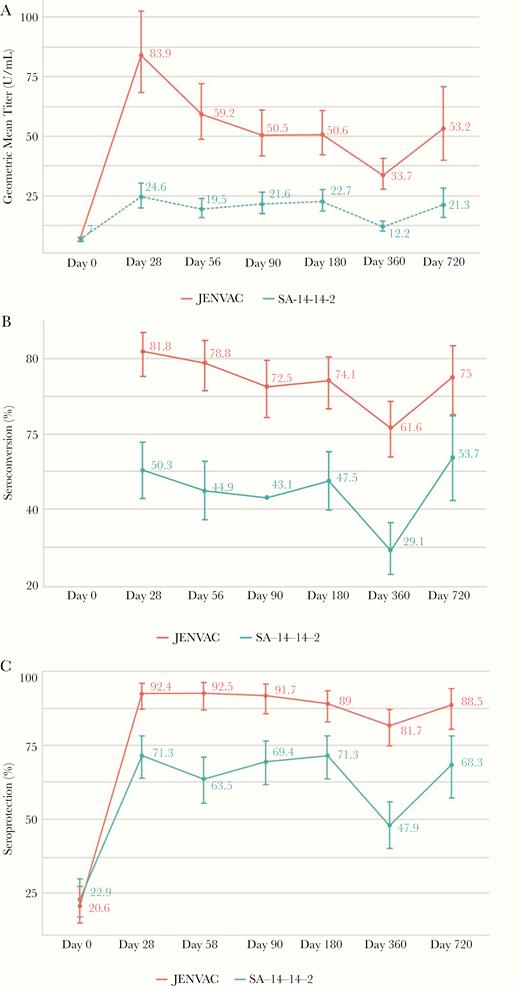
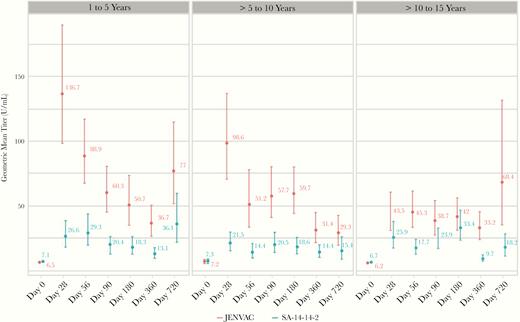
At day 360 (postvaccination), GMTs were 33.7 (95% CI, 27.9–40.77) and 12.2 (95% CI, 10.3–14.4) in the JENVAC and SA 14-14-2 groups, respectively, and 81.7% (95% CI, 74.9–87.3) in JENVAC group and 47.9% (95% CI, 40.1–55.8) in the SA 14-14-2 group achieved SPR (Figure 2). The GMT ratio for recipients (at day 28) of JENVAC at the first dose relative to the GMT for recipients of SA 14-14-2 was 2.91 (Table 2). JENVAC reported statistically higher immune responses at day 28 and subsequent time points. In the JENVAC-stratified age groups, the SPR at day 360 was 85.7% (95% CI, 76.6–94.9), 75.9% (95% CI, 64.5–87.3), and 83.3% (95% CI, 73.4–93.3) in the ≥1 to ≤5, 5> to ≤10, >10 to ≤15 years, respectively. Geometric mean titers at most time points in the JENVAC group was significantly higher than the SA 14-14-2 group (Figure 3).
Regression Modeling of Effects of Vaccine Given at First and Second Dose on Geometric Mean Titer and Seroconversiona
| Variable . | GMT Ratio (95% CI) . | P Value . | Odds Ratio SCR (95% CI) . | P Value . |
|---|---|---|---|---|
| JENVAC at 1st dose | 2.91 (1.84–4.59) | <.0001 | 7.02 (2.82–17.45) | <.0001 |
| JENVAC at 2nd dose | 1.69 (1.10–2.60) | .017 | 1.87 (.85–4.12) | .12 |
| Log10 titer at day 720 | 1.87 (1.30–2.70) | .017 | 0.10 (.05–.23) | <.0001 |
| Variable . | GMT Ratio (95% CI) . | P Value . | Odds Ratio SCR (95% CI) . | P Value . |
|---|---|---|---|---|
| JENVAC at 1st dose | 2.91 (1.84–4.59) | <.0001 | 7.02 (2.82–17.45) | <.0001 |
| JENVAC at 2nd dose | 1.69 (1.10–2.60) | .017 | 1.87 (.85–4.12) | .12 |
| Log10 titer at day 720 | 1.87 (1.30–2.70) | .017 | 0.10 (.05–.23) | <.0001 |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; SCR, seroconversion.
aIn a linear regression modeling of the log10-transformed titer at day 748 model with only the main effects of the 3 independent variables, all 3 were significant. The estimated GMT ratio for recipients of JENVAC at the first dose, relative to the GMT for recipients of SA 14-14-2 at the first dose, was 2.91. For the booster dose, the estimated GMT ratio for JENVAC recipients was 1.69. In the model for seroconversion at day 748 relative to the titer at day 720 as a baseline, the effects are presented as odds ratios and interpreted similarly as the effects in the model for log10 (titer at day 748), the effect of the JENVAC at first dose was significant (P < .0001), but the effect of the vaccine given at booster dose was not (P = .12). The estimated odds ratio of seroconversion for JENVAC recipients at the first dose, relative to the odds for SA 14-14-2 recipients at the first dose, was 7.02.
Regression Modeling of Effects of Vaccine Given at First and Second Dose on Geometric Mean Titer and Seroconversiona
| Variable . | GMT Ratio (95% CI) . | P Value . | Odds Ratio SCR (95% CI) . | P Value . |
|---|---|---|---|---|
| JENVAC at 1st dose | 2.91 (1.84–4.59) | <.0001 | 7.02 (2.82–17.45) | <.0001 |
| JENVAC at 2nd dose | 1.69 (1.10–2.60) | .017 | 1.87 (.85–4.12) | .12 |
| Log10 titer at day 720 | 1.87 (1.30–2.70) | .017 | 0.10 (.05–.23) | <.0001 |
| Variable . | GMT Ratio (95% CI) . | P Value . | Odds Ratio SCR (95% CI) . | P Value . |
|---|---|---|---|---|
| JENVAC at 1st dose | 2.91 (1.84–4.59) | <.0001 | 7.02 (2.82–17.45) | <.0001 |
| JENVAC at 2nd dose | 1.69 (1.10–2.60) | .017 | 1.87 (.85–4.12) | .12 |
| Log10 titer at day 720 | 1.87 (1.30–2.70) | .017 | 0.10 (.05–.23) | <.0001 |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; SCR, seroconversion.
aIn a linear regression modeling of the log10-transformed titer at day 748 model with only the main effects of the 3 independent variables, all 3 were significant. The estimated GMT ratio for recipients of JENVAC at the first dose, relative to the GMT for recipients of SA 14-14-2 at the first dose, was 2.91. For the booster dose, the estimated GMT ratio for JENVAC recipients was 1.69. In the model for seroconversion at day 748 relative to the titer at day 720 as a baseline, the effects are presented as odds ratios and interpreted similarly as the effects in the model for log10 (titer at day 748), the effect of the JENVAC at first dose was significant (P < .0001), but the effect of the vaccine given at booster dose was not (P = .12). The estimated odds ratio of seroconversion for JENVAC recipients at the first dose, relative to the odds for SA 14-14-2 recipients at the first dose, was 7.02.
Comparison of geometric mean titers (GMTs) (A), proportion of children achieving seroconversion (B), and seroprotection (C) between groups, before vaccine (day 0) and postvaccine administration (days 28, 56, 90, 180, 360, and 720). (A) The GMTs were calculated as the antilog of the mean of the log transformation of titers for all the children within a group. (B) Seroconversion was defined as a 50% plaque reduction neutralization titer (PRNT50) of >10 U/mL if the baseline titer was <10 U/mL or as a 4-fold increase if the baseline titer was >10 U/mL. (C) Seroprotection was defined as the proportion of children with a PRNT50 >10 U/mL. A significant difference was observed between JENVAC and SA 14-14-2 groups (P < .05) at the respective day represented with 95% confidence intervals.
Comparison of geometric mean titers among treatment groups stratified by age. Geometric mean titers are based on 50% plaque reduction neutralization titers represented with 95% confidence intervals. Age stratified groups were classified as (A) ≥1 to ≤5 years, (B) 5> to ≤10 years, and (C) >10 to ≤15 years.
Interchangeability Study
On the final follow-up visit (day 720), 178 children (96 and 82 in the JENVAC and SA 14-14-2 groups, respectively) were assigned (nonrandom) to receive a booster dose or interchanged to receive the other vaccine. Forty-six children received JENVAC + JENVAC, 50 children received JENVAC + SA 14-14-2, 46 children received SA 14-14-2 + JENVAC, and 36 children received SA 14-14-2 + SA 14-14-2 (Figure 1).
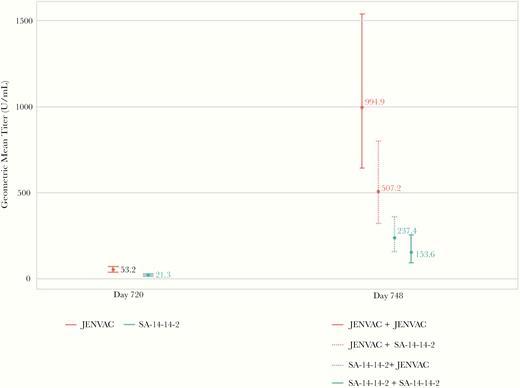
At day 720 (postreceipt of a single dose of either vaccine), 88.5% (95% CI, 80.4–94.1) in JENVAC group and 68.3% (57.1, 78.1) in the SA 14-14-2 group achieved SPR (Figure 2). The estimated GMT ratio for recipients of JENVAC at the first dose, relative to the GMT for recipients of SA 14-14-2 at the first dose, was 2.91 (P < .05) (Table 2). At day 748, the group that received JENVAC + JENVAC had the highest GMT response (Figure 4).
Comparison of geometric mean titers among boosted and interchanged treatment groups. Geometric mean titers are based on 50% plaque reduction neutralization titers represented with 95% confidence intervals.
Adverse Events
A total number of children reporting at least 1 AE was 57 and 62 in the JENVAC and SA 14-14-2 groups, respectively (Table 3). Fever was the most commonly reported solicited AEs with a reporting of 39.1% and 28.6% of events in the JENVAC and SA 14-14-2 groups, respectively. There was no significant difference between the JENVAC and SA 14-14-2 groups in either local or general AEs (P = .31). Two serious AEs were reported in the JENVAC group: 1 case of pneumonia and 1 case of urinary tract infection. Both events resolved within 1 week and were deemed unrelated to the vaccine. In the interchangeability study, 11 general AEs were reported (4, 2, and 5 in the JENVAC + JENVAC, JENVAC + SA 14-14-2, and SA 14-14-2 + JENVAC groups, respectively). No illness associated with JE was observed during the 3-year study period.
General Solicited Adverse Events by Treatment Group for the Trial Cohorta
| General Solicited AEs . | JENVAC (N = 180) n (%) . | SA 14-14-2 (N = 179) n (%) . |
|---|---|---|
| General Symptoms | ||
| Fever | 34 (39.1%) | 26 (28.6%) |
| Headache | 8 (9.2%) | 6 (6.6%) |
| Nausea | 1 (1.1%) | 1 (1.1%) |
| Vomiting | 3 (3.4%) | 5 (5.5%) |
| Others | 18 (20.7%) | 16 (17.6%) |
| Local Symptoms | ||
| Pain at injection site | 13 (14.9) | 17 (9.4) |
| Redness | 7 (8.0) | 11 (18.7) |
| Swelling | 3 (3.4) | 9 (9.9) |
| Total number of events | 87 (100%) | 91 (100%) |
| Number of children reporting at least 1 AE | 57 (31.7%) | 62 (34.6%) |
| General Solicited AEs . | JENVAC (N = 180) n (%) . | SA 14-14-2 (N = 179) n (%) . |
|---|---|---|
| General Symptoms | ||
| Fever | 34 (39.1%) | 26 (28.6%) |
| Headache | 8 (9.2%) | 6 (6.6%) |
| Nausea | 1 (1.1%) | 1 (1.1%) |
| Vomiting | 3 (3.4%) | 5 (5.5%) |
| Others | 18 (20.7%) | 16 (17.6%) |
| Local Symptoms | ||
| Pain at injection site | 13 (14.9) | 17 (9.4) |
| Redness | 7 (8.0) | 11 (18.7) |
| Swelling | 3 (3.4) | 9 (9.9) |
| Total number of events | 87 (100%) | 91 (100%) |
| Number of children reporting at least 1 AE | 57 (31.7%) | 62 (34.6%) |
Abbreviations: AE, adverse events; N, number of children; n, number of events in each group across visits; %, percentage of events in each group.
aComparison AEs between groups was performed using χ 2 test with Yate’s. Both groups showed comparable safety outcomes (P = .31).
General Solicited Adverse Events by Treatment Group for the Trial Cohorta
| General Solicited AEs . | JENVAC (N = 180) n (%) . | SA 14-14-2 (N = 179) n (%) . |
|---|---|---|
| General Symptoms | ||
| Fever | 34 (39.1%) | 26 (28.6%) |
| Headache | 8 (9.2%) | 6 (6.6%) |
| Nausea | 1 (1.1%) | 1 (1.1%) |
| Vomiting | 3 (3.4%) | 5 (5.5%) |
| Others | 18 (20.7%) | 16 (17.6%) |
| Local Symptoms | ||
| Pain at injection site | 13 (14.9) | 17 (9.4) |
| Redness | 7 (8.0) | 11 (18.7) |
| Swelling | 3 (3.4) | 9 (9.9) |
| Total number of events | 87 (100%) | 91 (100%) |
| Number of children reporting at least 1 AE | 57 (31.7%) | 62 (34.6%) |
| General Solicited AEs . | JENVAC (N = 180) n (%) . | SA 14-14-2 (N = 179) n (%) . |
|---|---|---|
| General Symptoms | ||
| Fever | 34 (39.1%) | 26 (28.6%) |
| Headache | 8 (9.2%) | 6 (6.6%) |
| Nausea | 1 (1.1%) | 1 (1.1%) |
| Vomiting | 3 (3.4%) | 5 (5.5%) |
| Others | 18 (20.7%) | 16 (17.6%) |
| Local Symptoms | ||
| Pain at injection site | 13 (14.9) | 17 (9.4) |
| Redness | 7 (8.0) | 11 (18.7) |
| Swelling | 3 (3.4) | 9 (9.9) |
| Total number of events | 87 (100%) | 91 (100%) |
| Number of children reporting at least 1 AE | 57 (31.7%) | 62 (34.6%) |
Abbreviations: AE, adverse events; N, number of children; n, number of events in each group across visits; %, percentage of events in each group.
aComparison AEs between groups was performed using χ 2 test with Yate’s. Both groups showed comparable safety outcomes (P = .31).
DISCUSSION
We report the safety and immunogenicity findings from a single dose of JENVAC and SA 14-14-2 and its interchangeability in children of ages 1–15 years, the most critical age group from JE disease burden point of view. All immunogenicity measures after JENVAC were found to be higher and longer-lived than the comparator SA 14-14-2. All age-stratified subgroups (≥1 to ≤5, 5> to ≤10, >10 to ≤15 years) demonstrated robust responses to JENVAC. Children receiving 2 doses of JENVAC (2 years apart) showed the highest GMT titer. The interchangeability study demonstrated that children who received their primary and booster vaccination with SA 14-14-2 had statistically lower JE-neutralizing antibodies compared with those receiving 2-doses of JENVAC.
Because JE vaccines are being widely used in India, conducting an efficacy trial with clinical outcomes was not feasible. According to the WHO consultation on immunological endpoints for JE vaccines, the accepted assay for protection is a serum-neutralizing antibody titer of at least 1:10 as determined by PRNT50 [20]. Immunogenicity analyses are influenced by the virus strain and cell substrate used in the PRNT50 assay. With no established reference serum, these immunogenicity results should therefore be considered in the context of the virus strain and cell substrate [3]. However, in the earlier phase 2/3 study, the authors evaluated the cross-reactivity of JENVAC sera with homologous/heterologous strains of JE virus. In addition, JENVAC sera was shown to variably cross-react with strains from genotypes I–IV in various cell substrates [8].
Before this study, the participants who dropped out (received 1 dose of either vaccine) from the phase 2/3 trial were contacted for collection of serum samples. Immune responses for JENVAC were higher even after 1 year postvaccination, further corroborating the persistence of antibody levels with a single dose [8]. Furthermore, the seroprotection rates in both the clinical trials post 1 dose of JENVAC were greater than 90%, establishing the fact that single dose of JENVAC is sufficient to give excellent immune protection against JE disease.
Findings comparing the SPRs (at day 28) of participants (same age groups) receiving JENVAC (single dose) were not comparable (99.3% and 92.4%, P = .0001) between the phase 2/3 and 4 studies, respectively. Both studies had spatial and temporal differences. Seroprevalence of JE (PRNT50 ≥10 U/mL on day 0) was 11.6% and 22.7% (P = .0002) between phase 2/3 (n = 397) and 4 (n = 359) studies, respectively, indicating a higher proportion of circulating JE antibodies in the latter study. National Vector Borne Disease Control Program (NVDCP) reported an enhanced number of JE cases and JE-related deaths, over the period during which the 2 clinical trials were carried out [21]. However, it is important to note that within the ages of 1–2 years, there was no difference in the seroprevalence at day 0 (P = .9), and the GMTs postvaccination with a single dose of JENVAC were also comparable, approximately 180 U/mL, suggesting that these children would not have had much exposure to subclinical JE infections. We believe that this enhanced baseline seroprevalence rate may have had an inverse relation to JE (JENVAC and SA-14-14-2) vaccine immune response in the older age groups (Supplementary Table 1).
In endemic countries, higher prevaccination dengue titers are known to result in lower JE postvaccination titers [22]. We hypothesize that the relatively lower postvaccination JE titers in the current study may be attributable to a heightened exposure to dengue, albeit it is well known that flavivirus antibodies cross-react with other flaviviruses [23, 24]. According to the NVDCP, from 2011 to 2019, there has been a marked increase in dengue cases and deaths [25, 26]. Most of the study sites are reported to be dengue-endemic regions.
This study reported an SPR of 92.4% (95% CI, 88.4–96.4) and 81.7% (95% CI, 75.8–87.6) at days 28 and 360, respectively, from a single dose of JENVAC. A more appropriate assessment would be to compare immune responses between JENVAC and other inactivated JE vaccines in endemic settings. An IXIARO study in the Philippines (endemic for JE) reported an SPR of 89.6% (95% CI, 80.0–94.8) 1 year after the primary immunization series (2 doses administered 4 weeks apart) [27]. Thus, a single dose of JENVAC offers similar long-term immune responses to that exhibited by 2 doses of the other inactivated JE vaccines.
Among participants who were lost to follow-up, the distribution among age groups and gender were comparable. We report that no potential biases (with regards to attrition) influenced the outcome of this study. A limitation of our study is that it was conducted in an endemic country, where JE virus is circulating, and, hence, natural exposure might influence the results. In JE-endemic areas, due to constant natural exposure to flaviviruses, the duration of protective responses (from a JE vaccine) is expected to be longer than that in nonendemic areas. In endemic countries, higher JE seroprevalence has been associated with persistence of antibodies towards JE [22]. An equal number of children in both groups (approximately 52%) and across all sites experienced natural boosting (based on higher immunogenicity titers on day 720 when compared with day 360), a proportion high enough to contribute to maintenance of responses (Figure 2). A similar phenomenon was not observed in our previous phase 2/3 trial in either group [8]. However, the clinical trials were carried out with a time gap of almost 4 years, and several of the sites were different.
According to the NVDCP, JE accounted for 16.2% of a total of 64 690 acute encephalitis syndrome cases reported during 2010–2017 [28]. In 2006, the SA 14-14-2 vaccine was introduced in India as part of the NIP in 181 endemic districts [16], which increased to 260 districts in 2017 [29]. It is now administered with 2 doses at 9 and 18 months of age, although it was initially introduced as a single-dose vaccine. Postmarketing surveillance by the Indian Council of Medical Research reported SCRs, 28 days postvaccination, to be 67.2% in JE-naive children alone and 73.9% overall. The vaccine effectiveness estimate is reported to be 43.8% (95% CI, 1.9–67.8) for a single dose of SA 14-14-2 [30]. Despite the vaccine being available for more than 12 years, numerous JE outbreaks were reported, affecting an increased proportion of adults and children [9, 31–36]. A few reasons for these recurring surges may be attributable to rapidly waning of JE-neutralizing antibodies conferred by the vaccine, emergence of new strains against which SA 14-14-2 does not offer protection, and suboptimal vaccine coverage for a 2-dose schedule. Such data are extremely critical before any JE vaccine is introduced into the country’s NIP.
Another limitation of our study is the absence of a control arm with no JE vaccine administration used [22]. This would ensure the assessment of the background JE seroprevalence rates as a function of time. However, the fact that we had an active comparator arm with SA 14-14-2 that bolsters the immune response patterns observed with JENVAC is a true reflection of the vaccine performance in an endemic setting. It has to be mentioned here that allocation of either vaccine on day 720 was not performed in a random manner. This was due to the uncertainty in recruiting adequate numbers after a 2-year follow-up period, thus introducing channeling bias. The investigators managed to recruit comparable numbers across groups (Figure 1).
It is currently recommended that inactivated and live JE vaccines be administered in 2 doses (4 weeks apart) as a primary immunization series [10, 11, 16], with the exception of IMOJEV [37]. A contributing factor to improving JE vaccine coverage would be limiting the number of doses, either in a primary immunization series or the number of booster doses required. It is estimated that approximately one third of the children (having no contraindications) visit a healthcare facility and miss 1 or more vaccines [38]. Japanese encephalitis vaccine coverage surveys conducted in India reported rates of 75% and 42% for the first and second dose, respectively [18]. A single-dose JE vaccine may be advantageous for mass vaccination. Further boosting at later time points may be beneficial. The Drug Controller of India has approved the licensure of JENVAC as a single dose in 2016.
CONCLUSIONS
Studies from new-generation JE vaccines are reporting longer lasting immunity with a primary immunization series (2 doses 4 weeks apart) [6, 8, 22, 39–41]. Our study reports the long-term persistence of JE-neutralizing antibodies with a single dose. There is a need for a shift in policy to adopt newer generation JE vaccines that show long-lasting immunity. Such a change would warrant interchangeability data. We demonstrated that children who received a primary vaccination and booster with SA 14-14-2 had significantly lower JE-neutralizing antibodies than children who received 2-doses of JENVAC. The ability to produce robust immune responses and elicit cross-protection against numerous homologous and heterologous JE strains from a single-dose provides JENVAC with the opportunity for global licensure. A single dose of JENVAC reported statistically higher immune responses 4 weeks postvaccination, proving to be an effective traveler’s vaccine. In future studied, researchers should evaluate the duration of protection of JENVAC for traveler’s in nonendemic settings.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Table 1. Regression Modeling of Effects of Baseline Seroprevalence and Age of Participant on JENVAC Seroconversion (SCR)
Notes
Acknowledgments. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Editors. We thank the children and their families who participated in this trial, all investigators, study coordinators, and nurses. We are grateful for the significant contributions by Siddharth Reddy, Vamshi Krishna, and Sandhya Nandala. Finally, we are grateful to Drs. Milind Gore and Nagendra Hegde for their review of this manuscript.
Disclaimer. Bharat Biotech International Limited (BBIL) was not involved in any stage of the conduct and analyses of this study.
Financial support. This work was funded by BBIL (CTRI/2014/02/004386 and CTRI/2016/05/006909). BBIL took charge of all the costs associated with the development and publishing of the present manuscript.
Potential conflicts of interest. V. K. and R. E. are employees of BBIL. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
JEEV [package insert]. India: Biological E. Ltd; 2015. Available at: https://www.who.int/immunization_standards/vaccine_quality/pq266_je_1dose_biologicale/en/. Accessed
IXIARO [package insert]. Austria: Valneva; 2018. Available at: https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm142569.pdf. Accessed
JEEV [package insert]. India: Biological E. Ltd; 2015. Available at: https://www.who.int/immunization_standards/vaccine_quality/pq_266_je_1dose_biologicale_insert_updated.pdf. Accessed
IMOJEV [package insert]. Thailand: Sanofi Pastuer; 2014. Available at: https://www.who.int/immunization_standards/vaccine_quality/PQ_277_JE_4dose_SP_PI.pdf. Accessed