-
PDF
- Split View
-
Views
-
Cite
Cite
Eugene S. Hurwitz, Michael Haber, Albert Chang, Timothy Shope, Siew T. Teo, Jill S. Giesick, Michele M. Ginsberg, Nancy J. Cox, Studies of the 1996–1997 Inactivated Influenza Vaccine among Children Attending Day Care: Immunologic Response, Protection against Infection, and Clinical Effectiveness, The Journal of Infectious Diseases, Volume 182, Issue 4, October 2000, Pages 1218–1221, https://doi.org/10.1086/315820
Close - Share Icon Share
Abstract
A randomized, blinded, pilot study of influenza vaccine administered to children attending day care centers was conducted during the 1996–1997 winter. Vaccine efficacy in preventing serologically proven influenza virus infection was 0.45 (95% confidence limit [CL]: −0.02, 0.69) for influenza B and 0.31 (95% CL: −0.95, 0.73) for influenza A(H3N2). For both influenza A(H3N2) and B, children without preexisting hemagglutination inhibition (HI) antibody to these antigens had lower antibody responses to vaccine, were less likely to develop a serological response, and were more likely to develop serological evidence of influenza infection. Although there were no reductions in respiratory or febrile respiratory illnesses among all vaccinated children, there was a trend for reductions in such illnesses among vaccinated children with preexisting HI antibodies to influenza A(H3N2) and B. Therefore, immunologic priming in young children may be important for vaccine response and for protection against infection. Larger studies are needed in other influenza seasons to assess vaccine efficacy and clinical effectiveness.
Recent interest has focused on the potential role of annual influenza vaccination of young children, including those attending day care, to prevent influenza-related morbidity, such as otitis media [1–3]. Studies used to establish recommendations for the dose and schedule of the inactivated influenza vaccine in young children were initially conducted in the late 1970s with A/New Jersey/76 and A/USSR/77 virus vaccines [4, 5]. None of these studies attempted to determine vaccine efficacy on the basis of prevention of laboratory-confirmed influenza or clinical influenza illnesses. Since these studies were conducted, there have been few studies of the currently recommended dosage or schedule of influenza vaccine in young children [6–9]. Here we describe the results of a blinded, placebo-controlled pilot study of the 1996–1997 influenza vaccine administered to children attending day care centers, including assessments of the immune response, efficacy in preventing infections (on the basis of serological studies), and clinical effectiveness.
Methods
Subjects, enrollment, and serological specimens
In September and October 1996, children 24–60 months old were recruited from 10 US Navy-affiliated day care centers (total eligible children, 748). Children of parents who agreed to participate were matched for 2 age groups (24–36 and 37–60 months) and were randomized to receive either influenza or hepatitis A (control children) vaccine. The vaccines were administered at the day care centers on specified vaccination days in September and November by nurses who were not blinded to the vaccine being administered. The nurses had been instructed not to provide this information to the parents. Blood specimens were obtained from the children before vaccination, 1 month after the second vaccination, and at the end of the study in April or May 1997 for subsequent testing for antibodies to influenza antigens.
Vaccine and schedule
The vaccines administered were the commercially available split virus influenza (Flushield purified subvirion; Wyeth-Lederle, Philadelphia) and hepatitis A (SmithKline Beecham, Philadelphia) vaccines in the standard recommended dose and schedule for age [10]. Only children who had not previously received an influenza vaccine and thus were given 2 doses 1 month apart as recommended were included in the analysis reported here. Children ⩽36 months old received 0.25 mL of vaccine; those >36 months old were given 0.5 mL of vaccine as recommended. All vaccines were administered intramuscularly, usually in the deltoid muscle. At the time of vaccination, parents were asked to monitor adverse events to vaccination by recording information on a standard form for 3 days after receipt of each vaccine. This information was obtained via telephone interview on days 4–10 after vaccination.
Antibody testing
Antibody testing to the vaccine antigens in the 1996–1997 influenza vaccine was done by standard hemagglutination inhibition (HI) assay after treatment of the human sera with a receptor-destroying enzyme (Denka, Tokyo) [11]. The HI titers reported here are the reciprocals of the last serum dilution to a maximum of 1280 that completely inhibited hemagglutination with the 3 vaccine antigens used in the HI tests: A/Nanchang/933/95(H3N2), A/Texas/36/91(H1N1), and B/Harbin/7/94 (ether treated).
Influenza infections and vaccine efficacy
Influenza infections were defined as a ⩾4-fold titer increase when comparing HI titers for specimens obtained 1 month after vaccination (vaccinated children) and before the influenza season (vaccinated and control children) with specimens obtained at the end of the study in May. Estimates of vaccine efficacy against infection were based on the incidence of serologically confirmed influenza infections among vaccinated and control subjects.
Clinical effectiveness of vaccine
Information concerning respiratory illnesses in children was obtained via telephone interviews every 2 weeks with the parents of the study children. Estimates of clinical effectiveness of vaccine in preventing illnesses and related events were based on rates of respiratory illnesses (any reported) and febrile respiratory illnesses (associated with a temperature of ⩾38.34°C and ⩾2 respiratory symptoms: fever, cough, sore throat, or runny nose lasting ⩾2 days) among vaccinated and control children during the influenza period. For both definitions, illnesses had to be preceded by ⩾3 symptom-free days before onset. In addition to respiratory illnesses, other respiratory-related events assessed included reported earaches, physician visits, diagnosed ear infections, antibiotic prescriptions, and days the child or parents missed day care or work, respectively, because of these illnesses. The influenza period was defined as the weeks during which influenza isolates were identified by active virological surveillance among children with respiratory illnesses visiting 1 of 4 pediatric clinics where children from the naval bases were likely to seek medical care.
Statistical analysis
Confidence limits (CLs) and P values for comparisons of vaccine efficacy and effectiveness between vaccinated and controls were calculated on the basis of the formula for the SE of the log of a risk ratio [12].
Results
Subject recruitment and demographics
In total, 150 (20%) of the children eligible to participate in this study were enrolled from 10 day care centers. Enrollment rates ranged from 10% to 25% of eligible children. During the study, 5 children were withdrawn: 2 were given influenza vaccine and 3 hepatitis A vaccine. Reasons for withdrawal included the development of influenza-like illness 1 day after vaccination and lack of interest. Of the remaining 145 children, 74 were randomized to receive influenza vaccine and 71 to receive hepatitis A vaccine. We give results for 60 influenza- and 67 hepatitis A—vaccinated children who received 2 doses of vaccine. Forty-six of the influenza-vaccinated and 51 of the hepatitis A—vaccinated (control) children had 3 serological specimens available for serological analysis. Sixty of the influenza-vaccinated and 65 of the control children had ⩾1 serological specimen available for the clinical effectiveness analysis. No statistically significant differences were observed by sex, race, or age of the children; 63% of the vaccinated children and 66% of the control children were ⩽36 months old. No significant differences were observed in the occurrence of adverse events among children who received either the first or second dose of influenza or hepatitis A vaccine [13].
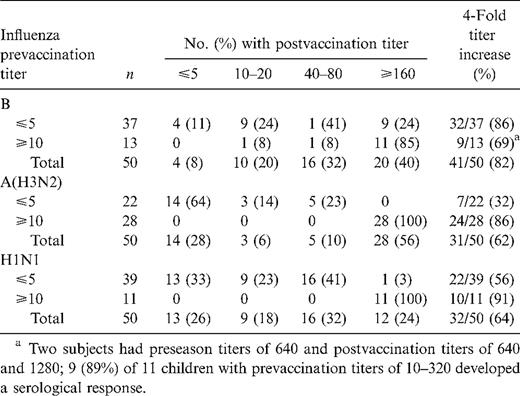
Preseason HI titer and response to influenza vaccine
Of the children tested, 66% had evidence of previous influenza A(H3N2) infection on the basis of a preseason HI titer ⩾10; 22% had antibody to influenza A(H1N1) and 26% to influenza B. Children with a detectable preseason HI titer ⩾10 to influenza A(H1N1) and A(H3N2) were significantly more likely to have a ⩾4-fold titer response to these antigens after vaccination than were those with a preseason HI titer ⩽5 (P = .0001 for A[H3N2]; P = .035 for A[H1N1]; table 1). Children with preseason HI titers ⩽5 to influenza A(H1N1) and A(H3N2) were less likely to develop a detectable antibody response to vaccine (HI titer ⩾10); 64% of children with HI titers ⩽5 to influenza A (H3N2) and 33% of children with HI titers ⩽5 to influenza A (H1N1) did not have any detectable HI antibody response to this antigen after vaccination. In addition, 85%–100% of children with preseason HI titers ⩾10 had postvaccination HI antibody titers ⩾ 160 to the 3 influenza antigens, compared with 0–24% of those with prevaccination titers ⩽5.
Serological response to influenza vaccine among children with and without prevaccination antibody
Children ⩾36 months old with prevaccination titers ⩽5 (all of whom received a larger dose of vaccine as recommended) were more likely to develop a serological response to the vaccine, a 4-fold titer increase, and a higher postvaccination HI titer than those <36 months old, although the numbers available for analysis were small, and no difference was statistically significant (table 1).
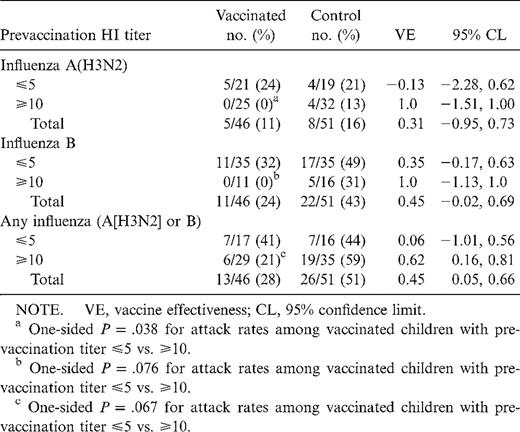
Influenza infections and vaccine efficacy against infection
Influenza activity began in mid-November, peaked in mid-January, and continued through early February (14 weeks). Only influenza A(H3N2) and influenza B isolates were obtained during this period. Vaccine efficacy was 0.31 for influenza A(H3N2), 0.45 for influenza B, and 0.45 for all influenza infections (table 2).
Vaccine efficacy stratified by prevaccination hemagglutination inhibition (HI) titers.
Because prevaccination HI titers were associated with serological response to vaccine and level of postvaccination titer, vaccine efficacy was estimated among those with HI titers ⩽5 and ⩾ 10. For both influenza A(H3N2) and B, the point estimate of efficacy was greater among those with prevaccination titers ⩾10 than among those with titers ⩽5, although only the estimate for any influenza infection (A[H3N2] or B) among those with prevaccination titers ⩾10 was statistically significant. In addition, for influenza A(H3N3) and B, influenza infections were more likely to occur in vaccinated children with prevaccination titers ⩽5 than in those with prevaccination titers ⩾10.
Clinical effectiveness
Although no significant vaccine effectiveness (VE) was observed against all respiratory illnesses (VE, −0.01; 95% CL: −0.16, 0.13) or febrile respiratory illnesses (VE, 0.07; 95% CL: −0.30, 0.23), there was a trend for greater clinical effectiveness among those with prevaccination titers ⩾10 to influenza A(H3N2), B, or both combined when compared with children with HI titers ⩽5. Among children with prevaccination HI titers ⩽5 to influenza A(H3N2) or B, the VE for respiratory illnesses was −0.23 (95% CL: −0.56, 0.03) versus 0.11 (95% CL: −0.09, 0.26) for those with prevaccination titers ⩾10 (VE comparison for those with titers ⩽5 and ⩾10, 2-sided P = .043). The VE for febrile respiratory illnesses was −0.20 (95% CL: −1.12, 0.33) for those with prevaccination HI ⩽5 versus 0.18 (95% CL: −0.24, 0.46) for children with titers ⩾10 (P = .26). There were no significant reductions in other respiratory illness-related events, including earaches, physician visits, diagnosed ear infections, antibiotics prescribed, or days the child or parent missed day care or work, respectively, because of the child's illness.
Discussion
In this study conducted with the vaccine formulation recommended for the 1996–1997 influenza season, previous exposure to influenza A and B viruses was an important predictor of immunological response to vaccine, postvaccination titer, and protection against infection. For both influenza A(H3N2) and B, protective responses to vaccine were more likely to occur in children with preexisting antibody, which suggests that immunological priming via previous exposure to influenza virus was an important factor in protection against serological infection.
Previous study results have suggested that young children with no detectable antibody to influenza may be less likely to develop an immunologic response to influenza vaccine than are children with preexisting antibody, although the specific influenza antigen to which children have had a reduced response has varied. In a study of children 6–36 months old during the 1978–1980 influenza season (with split-product vaccine administered in 2 doses 1 month apart), 11 of 22 children who had HI titers ⩽5 to influenza A(H1N1) developed an HI titer ⩾20, compared with that of all 7 children who had HI titers ⩾5 [8]. In contrast, 94% of children with titers ⩽5 to influenza A(H3N2) developed HI titers ⩾20, even after 1 dose of vaccine. In a second study conducted during 1980 among children and young adults 6 months to 22 years old, antibody response to influenza A(H1N1) was poorest for children 6 months to 6 years old, and initially seronegative subjects were less likely to develop an immune response (defined as an HI titer ⩾20) to both influenza A(H1N1) and B [9].
Our results suggest that a reduced or lack of immunologic response to influenza vaccine may occur with the currently recommended vaccine formulations. These results provide evidence that nonresponders to vaccine and those without pre-existing antibody are less likely to be protected against infection and are more likely to develop influenza infections, when defined as a 4-fold HI titer increase.
Assessment of the clinical effectiveness of influenza vaccine during the 14-week influenza period demonstrated no significant reductions in respiratory or febrile respiratory illnesses or in other respiratory-related events, including ear aches, physician visits, physician-diagnosed otitis media, or antibiotic use among vaccinated children. However, among those with prevaccination titers ⩾10, there was a trend for effectiveness that was greater than for those with titers ⩽5. This was consistent with serological analysis, which showed greater protection against infection among children with preexisting titers to influenza. The apparent lack of clinical effectiveness of influenza vaccine in this study in contrast to previous studies (particularly those showing reductions in otitis media [1–3]) may be related to a reduced efficacy of the 1996–1997 vaccine, to differing study methods, and to the small sample size. Another factor may have been the difficulty in distinguishing clinical influenza illnesses from the many other causes of respiratory illnesses in this age group. Because cultures for influenza were not obtained from study children with respiratory illnesses, no assessment of vaccine efficacy in reducing culture-confirmed illnesses, including otitis media, was possible.
The power of this pilot study to define vaccine efficacy and effectiveness was limited by the small sample size, which resulted in wide confidence estimates, particularly for vaccine efficacy. Additional studies of vaccines used in subsequent influenza seasons are needed to further evaluate the level of protection provided by influenza vaccine in immunologically naive children and to assess the clinical effectiveness of these vaccines in young day care—aged children. The recent outbreak of influenza A(H5N1) in Hong Kong and the threat of a pandemic of this or another new strain of influenza for which children may have no existing immunity [14] further emphasizes the need to determine the role and importance of priming by prior infection (or vaccination) in developing protection against infection and clinical illnesses, particularly in immunologically naive young children.
Acknowledgments
We thank the staff of the naval child development centers in San Diego and Camp Pendleton for assistance in conducting these studies, the interview staff and members of the San Diego Safe and Healthy Children in Childcare project, and the San Diego County Health Department Laboratory for support. We appreciate the scientific advice and input of Lawrence Schonberger, Robert Holman, and Aaron Curns, the administrative support of Voughn Trader, and the editorial assistance of John O'Connor (Centers for Disease Control and Prevention [CDC]). We also thank the CDC for primary funding and Wyeth-Lederle Vaccines and Pediatrics for partial funding of this study.
References
Human subject approval was obtained from the institutional review boards of the US Naval Medical Center, San Diego, Centers for Disease Control and Prevention (CDC), and San Diego State University School of Public Health. Written informed consent for participation of the children was obtained from their parents or legal guardians.
Financial support: CDC and Wyeth-Lederle Vaccines and Pediatrics.
The use of trade names or commercial sources is for identification only and does not imply endorsement by the US Department of Health and Human Services.