-
PDF
- Split View
-
Views
-
Cite
Cite
Jamie Harper, Ciaran Skerry, Stephanie L. Davis, Rokeya Tasneen, Mariah Weir, Igor Kramnik, William R. Bishai, Martin G. Pomper, Eric L. Nuermberger, Sanjay K. Jain, Mouse Model of Necrotic Tuberculosis Granulomas Develops Hypoxic Lesions, The Journal of Infectious Diseases, Volume 205, Issue 4, 15 February 2012, Pages 595–602, https://doi.org/10.1093/infdis/jir786
Close - Share Icon Share
Abstract
Background. Preclinical evaluation of tuberculosis drugs is generally limited to mice. However, necrosis and hypoxia, key features of human tuberculosis lesions, are lacking in conventional mouse strains.
Methods. We used C3HeB/FeJ mice, which develop necrotic lesions in response to Mycobacterium tuberculosis infection. Positron emission tomography in live infected animals, postmortem pimonidazole immunohistochemistry, and bacterial gene expression analyses were used to assess whether tuberculosis lesions in C3HeB/FeJ are hypoxic. Efficacy of combination drug treatment, including PA-824, active against M. tuberculosis under hypoxic conditions, was also evaluated.
Results. Tuberculosis lesions in C3HeB/FeJ (but not BALB/c) were found to be hypoxic and associated with up-regulation of known hypoxia-associated bacterial genes (P < .001). Contrary to sustained activity reported elsewhere in BALB/c mice, moxifloxacin and pyrazinamide (MZ) combination was not bactericidal beyond 3 weeks in C3HeB/FeJ. Although PA-824 added significant activity, the novel combination of PA-824 and MZ was less effective than the standard first-line regimen in C3HeB/FeJ.
Conclusions. We demonstrate that tuberculosis lesions in C3HeB/FeJ are hypoxic. Activities of some key tuberculosis drug regimens in development are represented differently in C3HeB/FeJ versus BALB/c mice. Because C3HeB/FeJ display key features of human tuberculosis, this strain warrants evaluation as a more pathologically relevant model for preclinical studies.
With few exceptions, current preclinical evaluation of new tuberculosis drug candidates in animals is limited to mice. Mice are relatively inexpensive and permit investigation of disease mechanisms given the wide availability of reagents. However, the major disadvantage of conventional mouse strains is the lack of necrotic (caseous) granuloma formation in response to infection by Mycobacterium tuberculosis. Caseous granulomas are the hallmark of human tuberculosis and may provide a unique bacterial microenvironment. Furthermore, unlike caseous tuberculosis granulomas in guinea pigs, rabbits, and nonhuman primates, tuberculosis granulomas in the conventional mouse strains are not hypoxic [1]. If caseation or an associated host microenvironment, such as hypoxia, is a determinant of bacterial persistence in human tuberculosis, it may be essential to perform tuberculosis studies and evaluate tools targeting mycobacterial persisters in animals that undergo caseation and develop hypoxic tuberculosis lesions [2].
Pan et al have reported that C3HeB/FeJ mice display lung pathology with central caseous necrosis [3, 4]. In this study, we used noninvasive positron emission tomography (PET) imaging in live animals and postmortem pimonidazole immunohistochemistry [1, 5, 6] and demonstrated that necrotic pulmonary tuberculosis lesions in chronically infected C3HeB/FeJ mice are hypoxic. Using quantitative reverse-transcription polymerase chain reaction (RT-PCR), we also demonstrated significant up-regulation of hypoxia-associated genes in bacteria obtained from these necrotic lesions. Finally, we evaluated the efficacy of combination tuberculosis drug therapies in this novel mouse model, including one regimen containing PA-824, a new tuberculosis drug candidate reported to be active against nonreplicating bacteria under hypoxic conditions [7, 8, 9].
MATERIALS AND METHODS
Animal Infections for Assessing Hypoxia
Four- to 6-week-old female BALB/c (Charles River) or C3HeB/FeJ (Jackson Laboratory) mice were aerosol infected with M. tuberculosis H37Rv using the Middlebrook Inhalation Exposure System (Glas-Col) with frozen titrated bacterial stocks. Mice were killed 1 day after infection. Their lungs were removed aseptically, homogenized, and plated for colony-forming unit (CFU) counts to determine the number of bacilli implanted. At 4 and 14 weeks (C3HeB/FeJ mice) or 2 and 8 weeks (BALB/c mice) after infection, 3–7 mice were killed to determine lung CFU counts or hypoxia.
[64Cu]ATSM PET Imaging
Copper(II)-diacetyl-bis(N4-methyl-thiosemicarbazone), or Cu-ATSM, is a noninvasive PET imaging tracer used to detect hypoxia in both preclinical and clinical setting [10, 11, 12]. Although it rapidly clears out of normoxic cells, Cu-ATSM is reduced and retained in live cells that are significantly hypoxic (partial pressure of oxygen [pO2] < 3.8 mm Hg) [13] and correlates with tissue pO2 [12, 14]. Live M. tuberculosis–infected animals were imaged within sealed biocontainment devices using methods described elsewhere [15, 16]. On the day of imaging, each mouse was weighed and injected with 100 mg/kg of pimonidazole HCl (Hypoxyprobe-1; HPI) using the intraperitoneal route. At the same time 250 μCi of [64Cu]ATSM was also injected via the tail vein. A 60-minute, dynamic PET acquisition sequence (five 2-minute frames, followed by ten 5-minute frames) and computed tomography (CT) were performed using the Mosaic HP PET (Philips) and NanoSPECT/CT (Bioscan) imagers, respectively. Positron emission tomography images were reconstructed and coregistered with CT images using Amide software (version 0.9.1) (http://amide.sourceforge.net). Spherical (1.5-mm diameter) regions of interest (ROIs) were traced around the tuberculosis lesions or randomly selected areas (control animals) in the lung images, making sure not to overlap the surrounding PET-active areas. At least 3 ROIs were assessed for each group and at each time point. The mean PET activities were computed by normalizing the ROI activities for each mouse to its thigh muscle activity (counts per minute per milligram of tissue) obtained post mortem using an automated gamma counter.
Pimonidazole Immunohistochemistry
After completion of imaging, and 1.5 hours after the pimonidazole administration, the imaged animals were killed and their organs were harvested and fixed. Hematoxylin-eosin histology and pimonidazole immunohistochemistry were performed on fixed and embedded lung and kidney (positive control) tissue samples, as described elsewhere [6].
Bacterial Gene Expression
Whole lungs from 10 C3HeB/FeJ mice were harvested 14 weeks after infection and immediately homogenized in 5 mol/L guanidine isothiocyanate lysis buffer (Sigma-Aldrich) [17]. After homogenization, samples were pelleted by centrifugation, and RNA was isolated using conventional methods [18]. RNA samples were reverse transcribed using gene specific primer, and quantitative RT-PCR was performed in triplicate using the iCycler system (Bio-Rad). Data were normalized to M. tuberculosis housekeeping gene sigA or 16s ribosomal RNA and expressed as fold change over a log-phase in vitro M. tuberculosis culture grown in Middlebrook 7H9 media (Difco).
Chemotherapy
Treatment with combination regimens began 8 weeks after low-dose aerosol infection of C3HeB/FeJ mice: standard first-line treatment regimen (RHZ) consisting of rifampin (R), isoniazid (H), and pyrazinamide (Z); 2 rifapentine (P)– or moxifloxacin (M)–containing regimens (PHZ and PMZ); PA-824 in combination with MZ (PaMZ) [19]; and MZ alone (control). Each regimen was administered for 12 weeks, except RHZ and PaMZ, for which 16-week treatment arms were also evaluated. Isoniazid (10 mg/kg), rifampin (10 mg/kg), pyrazinamide (150 mg/kg), moxifloxacin (100 mg/kg), rifapentine (10 mg/kg), or PA-824 (50 mg/kg) were administered by gavage 5 days per week. Pyrazinamide was administered only during the first 8 weeks. Untreated mice served as negative controls. At least 4 mice were killed from each group and time point assessed. Whole lungs from each animal were homogenized and plated for CFU counts. Additional cohorts of mice were held for 12 weeks after cessation of treatment to assess for culture positivity indicating relapse. Drug susceptibility was performed using agar proportion or doubling dilutions. All protocols were approved by the Johns Hopkins Biosafety, Radiation Safety, and Animal Care and Use committees.
Statistical Analysis
Statistical comparison between groups was performed using Student’s t test (2-tail distribution, 2-sample unequal variance) in Excel 2007 software (Microsoft), and χ2 was calculated using Prism 4 software, version 4.01 (GraphPad software). Data are presented on a logarithmic scale as means ± standard deviations for gene expression and CFU counts.
RESULTS
Evaluation of Hypoxia
To approximate the infection as it occurs in humans, we used a low-dose aerosol infection of C3HeB/FeJ mice, followed by several weeks of incubation. Bacterial CFU counts plateau in the lungs of C3HeB/FeJ mice 6–8 weeks after low-dose aerosol infection with M. tuberculosis. Discrete pulmonary lesions become apparent 4 weeks after infection and evolve into well-formed necrotic granulomas by 6–10 weeks [15]. To achieve a bacterial burden during the chronic phase that was similar to that in C3HeB/FeJ mice, a higher-dose aerosol infection was administered to BALB/c mice, in which bacterial growth plateau is achieved 2–3 weeks after infection. Pulmonary bacterial burdens for both mouse strains are shown in Table 1. To assess each mouse strain at similar points on the bacterial growth curve, C3HeB/FeJ mice were evaluated at 4 weeks (acute phase) and 14 weeks (chronic phase, 6–8 weeks after the plateau in bacterial CFU counts) and compared with BALB/c mice at 2 weeks (acute phase) and 8 weeks (chronic phase, 6–8 weeks after plateau in bacterial CFU counts) after infection. Equivalent pulmonary bacterial burdens were achieved in both mouse strains at these time points (P ≥ .80).
Pulmonary Bacterial Burdens in C3HeB/FeJ and BALB/c Mice After Aerosol Infection
| Mean ± SD, Log10 CFUs | |||
| Time Point | C3HeB/FeJ | BALB/c | P |
| Day 1 | 0.75 ± 0.15 | 2.25 ± 0.15 | |
| Acute phase | 4.83 ± 1.71 | 4.71 ± 0.20 | .92 |
| Chronic phase | 7.04 ± 1.09 | 6.93 ± 0.01 | .80 |
| Mean ± SD, Log10 CFUs | |||
| Time Point | C3HeB/FeJ | BALB/c | P |
| Day 1 | 0.75 ± 0.15 | 2.25 ± 0.15 | |
| Acute phase | 4.83 ± 1.71 | 4.71 ± 0.20 | .92 |
| Chronic phase | 7.04 ± 1.09 | 6.93 ± 0.01 | .80 |
These data are representative of multiple infections; ≥3 mice were used for each infection and time point. P values were calculated using Student’s t test (2-tail distribution, 2-sample unequal variance).
Abbreviations: CFUs, colony-forming units; SD, standard deviation.
Pulmonary Bacterial Burdens in C3HeB/FeJ and BALB/c Mice After Aerosol Infection
| Mean ± SD, Log10 CFUs | |||
| Time Point | C3HeB/FeJ | BALB/c | P |
| Day 1 | 0.75 ± 0.15 | 2.25 ± 0.15 | |
| Acute phase | 4.83 ± 1.71 | 4.71 ± 0.20 | .92 |
| Chronic phase | 7.04 ± 1.09 | 6.93 ± 0.01 | .80 |
| Mean ± SD, Log10 CFUs | |||
| Time Point | C3HeB/FeJ | BALB/c | P |
| Day 1 | 0.75 ± 0.15 | 2.25 ± 0.15 | |
| Acute phase | 4.83 ± 1.71 | 4.71 ± 0.20 | .92 |
| Chronic phase | 7.04 ± 1.09 | 6.93 ± 0.01 | .80 |
These data are representative of multiple infections; ≥3 mice were used for each infection and time point. P values were calculated using Student’s t test (2-tail distribution, 2-sample unequal variance).
Abbreviations: CFUs, colony-forming units; SD, standard deviation.
Noninvasive [64Cu]ATSM PET Imaging
The mean lung PET activity from dynamic acquisitions obtained from M. tuberculosis–infected mice is shown in Figure 1. No accumulation of [64Cu]ATSM was noted in either mouse strain during the acute phase of infection (Figure 1A). Mean 40–60-minute lesion-to-muscle ratios were 1.53 ± 0.50, 1.60 ± 0.14, and 1.41 ± 0.14 for the C3HeB/FeJ, BALB/c, and uninfected C3HeB/FeJ mice, respectively. In contrast, during the chronic phase, progressive time-dependent accumulation of [64Cu]ATSM was observed in the tuberculosis lesions of the C3HeB/FeJ mice with no accumulation in the control mice (BALB/c and uninfected C3HeB/FeJ) (P < .001) (Figure 1B). Mean 40–60-minute lesion-to-muscle ratios were 4.09 ± 0.40, 1.20 ± 0.17, and 0.72 ± 0.14 for the C3HeB/FeJ, BALB/c, and uninfected C3HeB/FeJ mice, respectively. [64Cu]ATSM PET and CT images from an infected C3HeB/FeJ mouse demonstrate colocalization of the PET signal with the tuberculosis lesion visualized with CT (Figure 2), CT and normalized PET images from a chronically infected BALB/c mouse show no areas of focused PET activity (Supplementary Figure 1).
Positron emission tomographic (PET) imaging demonstrates accumulation of hypoxia probe copper(II)-diacetyl-bis(N4-methyl-thiosemicarbazone) ([64Cu]ATSM) in tuberculosis lesions of C3HeB/FeJ mice. The mean [64Cu]ATSM PET lung activity normalized to the thigh muscles from dynamic acquisitions is shown for acute (A) and chronic infection (B) time points. During the chronic phase, progressive time-dependent accumulation of [64Cu]ATSM was observed in pulmonary tuberculosis lesions of infected C3HeB/FeJ mice (squares), with no accumulation observed in the infected BALB/c (triangles) and uninfected C3HeB/FeJ (X's) mice (P < .001). No [64Cu]ATSM accumulation was noted during the acute phase. Data are presented as means ± standard deviations.
Copper(II)-diacetyl-bis(N4-methyl-thiosemicarbazone) ([64Cu]ATSM) is localized to tuberculosis lesions of C3HeB/FeJ mice. Transverse, coronal, and sagittal computed tomographic (CT) and positron emission tomographic (PET) images from a Mycobacterium tuberculosis–infected C3HeB/FeJ mouse lung during the chronic phase of infection are shown. In the right panel (CT), the tuberculosis lesion is seen as a consolidation (gray) just posterior to the heart (H) (arrows). The middle panel (PET) shows the corresponding [64Cu]ATSM PET images. The arrows point to areas of high [64Cu]ATSM PET activity and the region of interest (encircled). The left panel (PET plus CT) shows the colocalization of the [64Cu]ATSM PET signal and the tuberculosis lesion seen on the CT images.
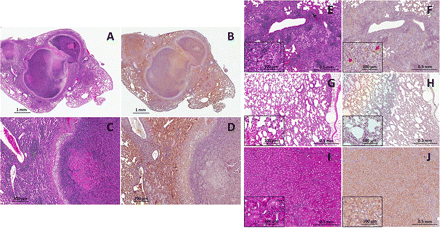
Postmortem Histopathology and Pimonidazole Immunohistochemical Analyses
Infected lung tissues from C3HeB/FeJ mice were compared with those obtained from infected BALB/c mice. Uninfected C3HeB/FeJ mice were used as negative controls, and kidney sections containing hypoxic renal tubular cells were used as positive controls [6]. Well-formed granulomas with central necrosis were observed in the lungs of C3HeB/FeJ mice (Figure 3A and 3C). Pimonidazole staining was noted around the periphery of these necrotic granulomas (Figure 3B and 3D). Although there was no histological evidence of necrosis, small foci of pimonidazole staining were observed in the chronically infected BALB/c mice (Figure 3E, F -inset). No pimonidazole staining was noted in the uninfected C3HeB/FeJ mice (Figure 3G, H), while significant pimonidazole staining was observed in the hypoxic renal tubular cells in the kidney tissues (Figure 3I, J). Because pimonidazole only accumulates in tissues with pO2 < 10 mm Hg [5], these data confirm the noninvasive imaging findings and indicate that the necrotic tuberculosis granulomas in the lungs of C3HeB/FeJ mice are significantly hypoxic.
Postmortem histopathology and pimonidazole immunohistochemical analyses. Hematoxylin-eosin histology (A, C, E, G, I) and pimonidazole immunohistochemistry (B, D, F, H, J) were performed on lung sections from chronically infected C3HeB/FeJ mice 14 weeks after infection (A–D), BALB/c mice 8 weeks after infection (E, F), and uninfected C3HeB/FeJ mice (G, H) and on renal tubular cells in kidney tissues from C3HeB/FeJ mice (I, J). Pimonidazole staining is noted around the periphery of the necrotic granulomas (B, D). Although there is no evidence of necrosis, small foci of pimonidazole staining is observed in the chronically infected BALB/c mice (F,inset). No pimonidazole staining is observed in the uninfected C3HeB/FeJ mice (H), whereas significant pimonidazole staining is noted in the renal tubular cells in the kidney tissues (J).
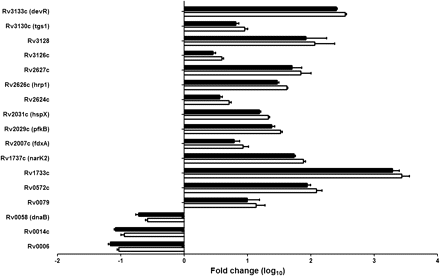
Bacterial Gene Expression in Response to Hypoxia
Bacterial transcriptional response for 14 hypoxia-associated genes known to be highly up-regulated in response to hypoxia and the enduring hypoxic response [20–23] were measured using quantitative RT-PCR. Three hypoxia-independent M. tuberculosis genes (Rv0006, Rv0014c, and Rv0058) were used as controls. M. tuberculosis isolated from the lungs of the chronically infected C3HeB/FeJ mice were compared with bacteria grown in vitro. Although none of the control genes were up-regulated, all hypoxia-associated M. tuberculosis genes were highly up-regulated in bacteria isolated from the lungs of chronically infected C3HeB/FeJ mice (P < .001) (Figure 4). Results were similar whether the data were normalized to M. tuberculosis sigA or 16s ribosomal RNA. These data are consistent with a bacterial response to the hypoxic host microenvironment and highlight that the necrotic tuberculosis granulomas in the lungs of C3HeB/FeJ mice are significantly hypoxic.
Quantitative real-time polymerase chain reaction (RT-PCR) for selected hypoxia-associated Mycobacterium tuberculosis genes. The bacterial transcriptional response for 14 hypoxia-associated and 3 hypoxia-independent genes (Rv0006, Rv0014c, and Rv0058) was measured by RT-PCR. M. tuberculosis isolated from the lungs of the chronically infected C3HeB/FeJ mice were compared with bacteria grown in vitro. Data are normalized to either sigA (open bars) or 16s ribosomal RNA (rRNA) (closed bars) and presented on a logarithmic scale as means ± standard deviations.
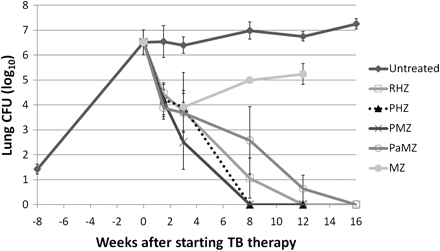
Efficacy of Combination Tuberculosis Drug Treatment
Low-dose aerosol infection implanted 1.42 ± 0.19 log10 CFUs in the lungs (Figure 5). Treatment began 8 weeks after infection, with a pulmonary bacterial burden of 6.52 ± 0.50 log10 CFUs. Lungs from mice treated with rifapentine containing regimens (PHZ and PMZ) were culture negative after 8 weeks of treatment, whereas mice receiving RHZ became culture negative only after 12 weeks of treatment. However, only 28% (2 of 7) and 83% (5 of 6) of mice treated with the novel PA-824 combination (PaMZ) were culture negative after 12 and 16 weeks of treatments, respectively. The culture-positive mouse (after 16 weeks of treatment) harbored PA-824–resistant bacteria. After displaying significant bactericidal activity over the first 3 weeks, MZ was largely ineffective. However, bacteria recovered from MZ-treated mice at the 12- and 16-week time points remained susceptible to moxifloxacin. No mouse receiving PHZ or PMZ for 12 weeks relapsed, whereas 20% (4 of 20) and 100% (8 of 8) of mice receiving RHZ and PaMZ, respectively, for 12 weeks relapsed (P = .0001 by χ2 test). After 16 weeks of treatment, 7% (1 of 14) and 70% (7 of 10) of mice receiving RHZ and PaMZ, respectively, relapsed (P = .0013 by χ2 test).
Efficacy of combination tuberculosis drug treatment in C3HeB/FeJ mice. Eight weeks after a low-dose aerosol infection, C3HeB/FeJ mice were allocated to different treatment groups. Lungs from mice treated with the PMZ and PHZ regimens were culture negative after 8 weeks of treatment, whereas lungs from mice treated with the standard RHZ regimen were culture negative only after 12 weeks of treatment. After displaying significant bactericidal activity during the first 3 weeks, MZ was ineffective and even permitted bacterial multiplication. Only 5 of 6 mice treated with PaMZ were culture negative after 16 weeks of treatment. At least 4 mice were killed for each group and time point assessed. Data are presented on a logarithmic scale as means ± standard deviations. CFUs, colony-forming units; H, isoniazid; M, moxifloxicin; P, rifapentine; Pa, PA-824; R, rifampin; Z, pyrazinamide.
DISCUSSION
Positron emission tomography is a functional imaging modality that relies on the detection of positrons emitted from radiolabeled tracers accumulating at the site of the diseased lesion and provides a comprehensive 3-dimensional assessment that closely correlates with overall disease process [24]. Several different PET tracers are in use and we have demonstrated elsewhere that [18F]-2-fluoro-deoxy-d-glucose PET correlates with bactericidal activity of tuberculosis drug treatments [15]. Because PET imaging is noninvasive, it can be used to study pathogenesis in live animals with relatively unaltered physiology. Although static spatial localization of lesions with PET has been extensively used to study disease processes, dynamic PET imaging can provide new insights into the temporal kinetics of tracer accumulation and has been used to study pharmacokinetics [25]. Because [64Cu]ATSM accumulates relatively quickly, 40–60-minute lesion-to-muscle ratios of ≥3.5 from dynamic PET acquisitions have been reported as an accurate cutoff for defining clinically significant hypoxia [26]. We therefore acquired several dynamic PET frames over 60 minutes. Although no significant accumulation of [64Cu]ATSM was observed in BALB/c mice, progressive and time-dependent accumulation was noted in the tuberculosis lesions of the chronically infected C3HeB/FeJ mice. Moreover, the mean 40–60-minute lesion-to-muscle ratio was ≥3.5 only for tuberculosis lesions in chronically infected C3HeB/FeJ mice (4.09 ± 0.40), indicating that they were hypoxic.
To confirm our findings, the same lesions were also evaluated using postmortem pimonidazole immunohistochemistry. Because live cells are required to reduce pimonidazole, the distribution of the staining was similar to what has been described elsewhere for tuberculosis granulomas—that is, mainly around the periphery of the central necrotic lesion [1]. Interestingly, small foci of hypoxia were detected in BALB/c mice during the chronic phase. Because BALB/c mice did not develop necrosis in response to M. tuberculosis infection, we hypothesize that these small foci may represent microscopic areas of limited oxygen diffusion due to edema or inflammation.
Because [64Cu]ATSM and pimonidazole demonstrate hypoxia in host cells, we also used bacterial transcriptional analysis as a host-independent method to evaluate hypoxia. Fourteen M. tuberculosis hypoxia-associated genes that are also members of the “enduring hypoxic response” described by Rustad et al [23] were evaluated and compared with 3 control (hypoxia-independent) genes. Although none of the control genes were found to be up-regulated, all hypoxia-associated M. tuberculosis genes were found to be significantly up-regulated in bacteria isolated from the necrotic tuberculosis lesions in C3HeB/FeJ mice. These hypoxia-associated genes include the dosR/devR regulon, thought to be the primary trigger in the metabolic shift-down to achieve dormancy [23]. devR was highly up-regulated in the bacteria obtained from the necrotic granulomas. In addition, Rv1733c, a part of the dosR/devR regulon, was most highly up-regulated. It is interesting to note that latently infected individuals mount strong T-cell responses to the protein encoded by Rv1733c [27].
In keeping with results reported elsewhere [20], α-crystallin coding gene hspX was also highly up-regulated in bacteria isolated from the necrotic tuberculosis lesions in C3HeB/FeJ mice. Timm et al have reported the expression of selected M. tuberculosis genes from bacteria isolated from in vitro cultures, lungs of C57BL/6 mice, and lung specimens from patients with active tuberculosis disease [28]. In their study, hspX was highly expressed in bacteria obtained from human samples compared with those obtained from C57BL/6 mice. Using whole genome microarray analyses, Talaat et al have identified genes up-regulated in BALB/c and SCID mice compared with an in vitro culture [29]. Although the number of hypoxia-associated genes evaluated in our study is small (n = 14), only fdxA was also found to be up-regulated in BALB/c and SCID models. These data further support the idea that tuberculosis lesions in C3HeB/FeJ strain are hypoxic and present a microenvironment different from that observed in conventional mouse strains.
Because tuberculosis lesions in C3HeB/FeJ mice display pathology and hypoxia similar to that observed in human tuberculosis [30], this strain warrants evaluation as a new, more pathologically relevant murine model for preclinical tuberculosis studies. We therefore evaluated the efficacy of tuberculosis drug regimens shown elsewhere to have promising activity in BALB/c mice. Consistent with findings of other studies [15, 31, 32], rifapentine-containing regimens were more potent than RHZ. However, although MZ produced a substantial early bacterial kill, it did not exert bactericidal activity beyond the first 3 weeks (Figure 5). This is contrary to the sustained bactericidal activity observed with MZ in BALB/c mice [19]. Similarly, although the addition of PA-824 increased the bactericidal and sterilizing activity of MZ, the PaMZ combination was less effective than RHZ. This is again contrary to the superior sterilizing activity of PaMZ compared with RHZ in BALB/c mice [19]. It should be noted that PaMZ is currently being compared with RHZ regimen (plus ethambutol) in a phase 2 trial. The reason for the apparent discrepancy in the activity of MZ with or without PA-824 relative to RHZ in C3HeB/FeJ versus BALB/c mice remains unclear.
The serum pharmacokinetics of moxifloxacin, pyrazinamide, and PA-824 in C3HeB/FeJ mice were similar to those observed in BALB/c mice (data not shown). However, the distribution of drugs inside the lesions themselves and the bacilli within the lesions were not measured. In the cellular aggregates that constitute the lesions of BALB/c mice, the bacilli reside intracellularly. Necrotic granulomas in C3HeB/FeJ mice (as in human granulomas) harbor both intracellular (macrophages within the granuloma wall) and extracellular (central necrotic core of the granuloma) bacilli, and differences in drug penetration into cells and/or the central necrotic core could account for differences in drug effects observed between these 2 strains. For example, recent studies in rabbits suggest that, although moxifloxacin is concentrated in the peripheral (cellular) regions of the tuberculosis granuloma, its penetration to the center of caseous lesions is restricted [33]. These questions will be an active area of research for our future studies. Finally, the following limitations of the current study must be noted. First, PaMZ and RHZ were compared in BALB/c mice after higher-dose aerosol infection and a 14-day incubation period between infection and treatment initiation [19], whereas the comparison in C3HeB/FeJ mice followed low-dose aerosol infection and an 8-week incubation period. Second, the PA-824 dose was 100 mg/kg in the BALB/c experiments, whereas 50 mg/kg was used in the current study to better reproduce the serum concentration–time profile after a 200-mg human dose.
In summary, our results support that the necrotic tuberculosis lesions in C3HeB/FeJ mice are hypoxic and that [64Cu]ATSM PET imaging can be used as a noninvasive, real-time method for evaluating M. tuberculosis–induced hypoxic lesions in situ. We have also demonstrated that that the activity of some tuberculosis drugs may be represented differently in C3HeB/FeJ mice. Therefore, C3HeB/FeJ mice warrant further evaluation as another more pathologically relevant murine model to determine whether they may better predict the efficacy of new tuberculosis drug and regimen candidates in humans.
Notes
Acknowledgments.
The authors wish to acknowledge Janine Knudsen and Sridhar Nimmagadda for their assistance in processing samples and imaging probe synthesis, respectively.
Financial support.
This work was supported by the National Institute of Health’s Director’s New Innovator Award (grant OD006492) and Bill and Melinda Gates Foundation TB Drug Accelerator grants (grants 48793 and 42851).
Potential conflicts of interest.
E. L. N. receives grant funding from sanofi-aventis, Global Alliance for TB Drug Development, Otsuka Pharmaceuticals, and Pfizer. S. K. J. and E. L. N. received travel/accommodation costs from sanofi-aventis for a nonpromotional lecture at the Satellite Symposium, 40th Union World Conference on Lung Health (December 2009; Cancun, Mexico). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: 48th Annual Meeting of the Infectious Diseases Society of America, Vancouver, British Colombia, Canada, October 2010 (abstract 4033); Tuberculosis: Immunology, Cell Biology and Novel Vaccination Strategies (J3), Mycobacteria: Physiology, Metabolism and Pathogenesis - Back to the Basics (J4) Keystone Symposium. January 15–20, 2011; Vancouver, British Columbia, Canada. (abstract 214).
J. H. and C. S. contributed equally to the study.




![Positron emission tomographic (PET) imaging demonstrates accumulation of hypoxia probe copper(II)-diacetyl-bis(N4-methyl-thiosemicarbazone) ([64Cu]ATSM) in tuberculosis lesions of C3HeB/FeJ mice. The mean [64Cu]ATSM PET lung activity normalized to the thigh muscles from dynamic acquisitions is shown for acute (A) and chronic infection (B) time points. During the chronic phase, progressive time-dependent accumulation of [64Cu]ATSM was observed in pulmonary tuberculosis lesions of infected C3HeB/FeJ mice (squares), with no accumulation observed in the infected BALB/c (triangles) and uninfected C3HeB/FeJ (X's) mice (P < .001). No [64Cu]ATSM accumulation was noted during the acute phase. Data are presented as means ± standard deviations.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/205/4/10.1093_infdis_jir786/2/m_infdisjir786f01_ht.gif?Expires=1716466725&Signature=MOiXDAc8YNoofkvd8owqsAUek-5bkn9ruHPcorUitZnDu4UNF5ar~nzb3hTkKZWAA3akUqbAVfYtdZPmB5dDetsI0jUfofb9Jo7bon~kzfxm-ls3uk7Gu4YypFXkFOjzl4jKHwdcdMtjOh3egq3dQh0xackDKmm6bt4xCzFBsogigR7y43p8-SBVGkY34mrgUg86XudOHiOEfB-wHMr7WScnsb5DtiHyDZ1bfWlGAF9Re0qFSvbEyK~5Wkpn7kdy~GHKh7D4aqVFpbygka7XaG8offB0gB7IFn83Q7j7hoYi5oowkWrS1GrVq6h2oDyzZIPaAqhNkX8gMAPbAx7LlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Copper(II)-diacetyl-bis(N4-methyl-thiosemicarbazone) ([64Cu]ATSM) is localized to tuberculosis lesions of C3HeB/FeJ mice. Transverse, coronal, and sagittal computed tomographic (CT) and positron emission tomographic (PET) images from a Mycobacterium tuberculosis–infected C3HeB/FeJ mouse lung during the chronic phase of infection are shown. In the right panel (CT), the tuberculosis lesion is seen as a consolidation (gray) just posterior to the heart (H) (arrows). The middle panel (PET) shows the corresponding [64Cu]ATSM PET images. The arrows point to areas of high [64Cu]ATSM PET activity and the region of interest (encircled). The left panel (PET plus CT) shows the colocalization of the [64Cu]ATSM PET signal and the tuberculosis lesion seen on the CT images.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/205/4/10.1093_infdis_jir786/2/m_infdisjir786f02_3c.gif?Expires=1716466725&Signature=UA6jTsl1luHNtqTdx92rEhMBFFgAl~ecjSVXjDXlJat83NnKM1LrxkVtuJN2J8iCi5Hwsurr24SMMDKItWLZzfo2EOP4jiNVq2VAOKJZ3DcfcgqO-DFt0UHEIqk4esHhFSwpxZ5UTb~9R0wmJ1g~Ol7ORZM51ZG6QGxny4m6ydYrEQYH9~VuFxJ7iDBYxv-EdLz0PTzb3CASxG4J1BGF7hZWlwJEibBs03Sx64kAjuBY1gAAnDCnxmvYOdb6Qs1CjdkTD8XFHPKqRg552bvAnKiu6wUVJzB-aQtTvhNmvfxZmjkFPhrT~tY26KkRc70ph5biYZhP3e8qyU1NvPmYMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)