-
PDF
- Split View
-
Views
-
Cite
Cite
Cristiana Gioia, Chiara Agrati, Concetta Castilletti, Maria R. Capobianchi, Federico Martini, Influenza Pandemics, Immune Cross-Reactivity, and Pandemic Control Strategies, The Journal of Infectious Diseases, Volume 198, Issue 2, 15 July 2008, Pages 294–295, https://doi.org/10.1086/589302
Close - Share Icon Share
To the Editor—In the 15 January 2008 issue of the Journal, Andreasen et al. show that in the 1918–1919 A/H1N1 influenza pandemic in Copenhagen, the lethal fall wave was preceded by a summer wave that was caused by a precursor pandemic virus that was transmitted efficiently but was not extremely virulent [1]. Interestingly, when preceded by this mild summer wave, the fall wave was less lethal, suggesting that the low transmissibility of the fall wave may be explained by partial cross-protection from subsequent summer exposure to a related influenza virus.
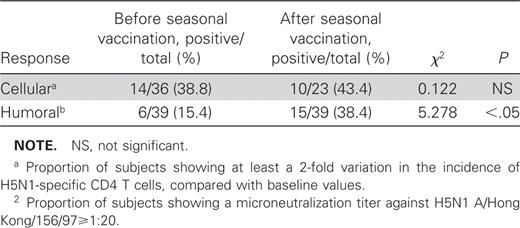
We have recently shown that a proportion of healthy subjects display cross-reacting humoral and cellular immune responses between human (H3N2/H1N1) and avian HPAI (H5N1) influenza strains [2]. In particular, a low incidence of CD4 T cells specific for H5N1 was detected in several persons at baseline, and seasonal vaccine administration enhanced the incidence of reactive CD4 T cells. We also observed that seasonal vaccination was able to raise the level of neutralizing antibodies against influenza (H5N1) in a large number of subjects (table 1). These findings highlight the possibility of boosting cross-type cellular and humoral immunity against the highly pathogenic avian influenza A virus subtype H5N1 by using seasonal influenza vaccination.
Proportion of healthy individuals showing cellular and humoral response to avian HPAI (H5N1) influenza strain before and after seasonal influenza vaccination (H3N2/H1N1).
A similar cross-reactive immunity was previously shown in animal models, which resulted in protection against H5N1 viral challenge. In particular, Lu et al. [3] studied influenza vaccines based on H5 hemagglutinin from a nonpathogenic avian influenza virus for the ability to induce cross-reactive immunity and/or cross-protection against a highly pathogenic H5N1 strain. These vaccines provided cross-protection from systemic infection, severe disease, and death after lethal challenges with H5N1 virus. Substantial levels of serum anti-H5 IgG were detected in mice that received vaccine. Moreover, Ichinohe et al. [4] evaluated the ability of currently licensed seasonal influenza vaccine to confer cross-protection against highly pathogenic H5N1 influenza virus in mice. Vaccinated mice manifested cross-reactivity of mucosal IgA and serum IgG with H5N1 virus, as well as both reduced H5N1 virus titers in nasal-wash samples, and an increased survival rate after challenge with H5N1 virus. Finally, Sandbulte et al. [5], by using DNA vaccination against the neuraminidase of a human H1N1 strain, showed an elicited serum IgG response to human N1 and robust protection against challenge with the homologous virus. Interestingly, vaccinated mice were partially protected from lethal challenge with the H5N1 virus; serum samples transferred from vaccinated mice to vaccination-naive animals conferred similar protection against mortality due to H5N1.
Moreover, mathematical models have shown that for diseases with antigenic drift, cross-reactive response not only may protect the population through classical herd immunity, but may reduce the chance of new variants being produced by limiting the number of affected subjects. Subsequent epidemics may therefore be milder as a result of this positive feedback [6]. The retrospective epidemiological data on 1918–1919 A/H1N1 influenza dual wave [1], as well as our data on vaccinated healthy persons [2], strongly suggest that cross-reactive immunity may also be protective in humans.
Overall, these data support the hypothesis that the cross-protective immunity induced by previous infections, sustained by circulating influenza virus strains, and/or boosted by seasonal vaccination may play an important role in the protective response against emerging influenza A infections waves. The data also shed light on the possible use of cross-protective immunity in pandemic control strategi
References
Potential conflicts of interest: none reported.