-
PDF
- Split View
-
Views
-
Cite
Cite
Lydia L Shook, Sara Brigida, James Regan, James P Flynn, Abbas Mohammadi, Behzad Etemad, Molly R Siegel, Mark A Clapp, Jonathan Z Li, Drucilla J Roberts, Andrea G Edlow, SARS-CoV-2 Placentitis Associated With B.1.617.2 (Delta) Variant and Fetal Distress or Demise, The Journal of Infectious Diseases, Volume 225, Issue 5, 1 March 2022, Pages 754–758, https://doi.org/10.1093/infdis/jiac008
Close - Share Icon Share
Abstract
There is limited information on the specific impact of maternal infection with the SARS-CoV-2 B.1.617.2 (delta) variant on pregnancy outcomes. We present 2 cases of intrauterine fetal demise and 1 case of severe fetal distress in the setting of maternal infection with delta-variant SARS-CoV-2. In all cases, fetal demise or distress occurred within 14 days of COVID-19 diagnosis. Evaluation revealed maternal viremia, high nasopharyngeal viral load, evidence of placental infection with delta-variant SARS-CoV-2, and hallmark features of SARS-CoV-2 placentitis. We suggest that delta-variant SARS-CoV-2 infection during pregnancy warrants vigilance for placental dysfunction and fetal compromise regardless of disease severity.
(See the Brief Report by Guan et al, on pages 748–53; See the Editorial Commentary by DeBiasi, on pages 745–7; See the Major Article by Regan et al, on pages 759–67.)
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection acquired during pregnancy is associated with an increased risk of adverse pregnancy outcomes including preeclampsia and preterm birth, particularly in cases of severe and critical maternal illness [1]. Although fetal growth restriction and stillbirth have been associated with some cases of maternal SARS-CoV-2 infection [1, 2], the extent to which SARS-CoV-2 infection itself might drive placental dysfunction leading to these pregnancy outcomes is not known, as a characteristic histopathological signature associated with maternal SARS-CoV-2 infection was not clearly identified in initial reports [3, 4]. Ultimately, an increased risk of stillbirth associated with maternal SARS-CoV-2 infection had not been consistently observed prior to the emergence of new variants of concern [5].
In April 2021, the Royal College of Physicians of Ireland and the Institute of Obstetricians and Gynaecologists issued a statement warning the public of 6 cases of stillbirth and 1 case of second trimester pregnancy loss associated with “SARS-CoV-2 placentitis”; results indicated a temporal link of this placental condition with the emergence of the B.1.1.7 (alpha) variant [6]. However, no other groups identified increased risk of adverse fetal outcomes associated with the alpha variant, which was rapidly eclipsed by the B.1.617.2 (delta) variant. Recently published data from the Centers for Disease Control and Prevention (CDC) demonstrate a 4-fold increased risk of stillbirth in deliveries with coronavirus disease 2019 (COVID-19) relative to those without COVID-19 during the period of delta predominance in the United States, a sharp increase when compared with prior epochs in the pandemic, raising concerns that delta-variant infection may be contributory [7]. This population-level study was not able to shed light on potential pathogenesis of the stillbirths, and scant information exists on the impact of SARS-CoV-2 variants on placental histopathology, although increased severity of maternal disease with delta-variant COVID-19 in pregnancy has been observed [8, 9]. Here we present 2 cases of intrauterine fetal demise and 1 case of fetal distress requiring emergent cesarean delivery in the setting of confirmed maternal infection with the SARS-CoV-2 delta variant, via sequencing of viral RNA isolated from the placenta, nasopharyngeal swabs, and maternal blood, with placental histopathologic features consistent with SARS-CoV-2 placentitis.
METHODS
All 3 participants were enrolled in the COVID-19 in Pregnancy Biorepository, approved by the Mass General Brigham Institutional Review Board (No. 2020P003538). All participants provided informed consent. Cases occurred in August 2021 at Massachusetts General Hospital (MGH), after the emergence and subsequent predominance of the B.1.612.7 variant in the Massachusetts community. For reference, during the month of August 2021, 348 deliveries were performed at MGH, and all were screened for SARS-CoV-2 at the time of delivery per hospital policy. Seven pregnant women who delivered in August tested positive for SARS-CoV-2 in late July and August, when the B.1.612.7 variant was likely the predominant variant in Massachusetts, including the 3 reported here.
At the time of delivery, placental biopsies were collected from the maternal and fetal sides of the placenta. Maternal plasma and nasopharyngeal swabs were also collected on admission to the hospital for delivery. Total RNA was extracted from placental homogenates, plasma samples, and nasopharyngeal swabs, and SARS-CoV-2 viral load was quantified using the US CDC 2019-nCoV_N1 primers and probe set as previously described [3]. The limit of quantification for viral load was 40 SARS-CoV-2 RNA copies/mL and positive values below that were set at 1.0 log10 RNA copies/mL. SARS-CoV-2 RNA extracted from placenta homogenates, plasma, and nasopharyngeal swabs was used as a template to amplify and sequence the viral genome. Sequencing was done using the QIAseq SARS-CoV-2 Primer Panel (Qiagen) as instructed by the manufacturer’s manual. Polymerase chain reaction (PCR) products were purified with AMPure XP beads (Beckman Coulter) and subsequently sequenced with the Illumina MiSeq sequencer, utilizing V2 chemistry. Raw Illumina reads were trimmed, and sequences were assembled by Sequencher 5 (Gene Codes Corp) and QIAGEN CLC Genomics Workbench (Qiagen). SARS-CoV-2 region-specific PCR amplification and sequencing was performed for regions with suboptimal sequencing coverage.
Placentas were sent for full pathologic examination and were examined grossly and sampled per the Amsterdam Consensus recommendations. At least 4 sections of placenta were sampled (umbilical cord, membranes, and at minimum 3 sections of parenchyma). Samples were fixed in formalin, processed, embedded, and stained with hematoxylin and eosin (H&E) for histopathologic examination per routine. One parenchymal section was chosen to study SARS-CoV-2 by RNA in situ hybridization (RNA-ISH) as previously described [10]. An experienced perinatal pathologist (D. J. R.) reviewed the gross and microscopic pathology, interpreted the RNA-ISH, and provided diagnoses.
RESULTS
Cases
Table 1 illustrates clinical case information for the 3 patients included in this report. Maternal age ranged from 30 to 39 years, all SARS-CoV-2 infections were mild in severity per National Institutes of Health criteria, all SARS-CoV-2 infections occurred in the third trimester (gestational age at infection 30 to 35 weeks), and all patients were unvaccinated. Two cases (case 1 and case 3) resulted in intrauterine fetal demise, and 1 (case 2) in a live birth after emergent cesarean delivery for category 3 fetal heart tracing. The number of days from initial COVID-19 symptoms to pregnancy outcome ranged from 5 to 10.
Case Demographic and Clinical Characteristics
| Characteristic . | Case 1 . | Case 2 . | Case 3 . |
|---|---|---|---|
| Maternal age, y | 37 | 30 | 39 |
| Gravidity and parity | G4P2012 | G1P0 | G6P5005 |
| Race | Other | White | Black |
| Ethnicity | Hispanic | Non-Hispanic | Non-Hispanic |
| Insurance | Private | Public | Public |
| Pregnancy outcome | Fetal demise | Live birth | Fetal demise |
| Gestational age at delivery or diagnosis of fetal demise | 32 weeks 6 days | 31 weeks 2 days | 37 weeks 1 day |
| Mode of delivery | Vaginal delivery | Cesarean delivery | Vaginal delivery |
| Pregnancy-related complications | GDMA1 | None | None |
| Prepregnancy BMI, kg/m2 | 22.3 | 32.7 | 23.3 |
| Medications | PNV | PNV, amoxicillin | PNV, protonix |
| TORCH serologies | Toxoplasmosis IgM−/IgG−; parvovirus IgM−/IgG+; CMV IgM−/IgG+; syphilis negative | Not performed | Toxoplasmosis IgM−/IgG−, parvovirus IgM−/IgG+; CMV IgM−/IgG+; syphilis negative |
| Cell-free DNA screening results | Low risk | Low risk | Low risk |
| Neonatal/fetal characteristics | |||
| Birthweight, g | 1395 | 1710 | 2535 |
| Birthweight percentilea | 8 | 73 | 21 |
| Length, cm | NA | 46 | |
| Head circumference, cm | NA | 30 | NA |
| Apgar score at birth | 0,0 | 4,7 | 0,0 |
| Sex | Female | Female | Female |
| Neonate or fetus tested for SARS-CoV-2? | Not tested | Yes, negative | Not tested |
| COVID-19 characteristics | |||
| Gestational week at SARS-CoV-2 infection | 31 | 30 | 35 |
| Severity of SARS-CoV-2 illnessb | Mild | Mild | Mild |
| Symptoms | Fever, cough, body aches, rhinorrhea | Fever, cough, sore throat | Fever, cough, muscle aches |
| Days from initial COVID-19 symptoms to pregnancy outcome | 9 | 5 | 10 |
| COVID-19 vaccination status | Unvaccinated | Unvaccinated | Unvaccinated |
| SARS-CoV-2 plasma viral load, log10 RNA per mL | 1.0c | NA | 1.0c |
| SARS-CoV-2 nasopharyngeal viral load, log10 RNA per mL | NA | 7.4 | 5.5 |
| Variant identified | B.1.617.2 | B.1.617.2 | B.1.617.2 |
| Characteristic . | Case 1 . | Case 2 . | Case 3 . |
|---|---|---|---|
| Maternal age, y | 37 | 30 | 39 |
| Gravidity and parity | G4P2012 | G1P0 | G6P5005 |
| Race | Other | White | Black |
| Ethnicity | Hispanic | Non-Hispanic | Non-Hispanic |
| Insurance | Private | Public | Public |
| Pregnancy outcome | Fetal demise | Live birth | Fetal demise |
| Gestational age at delivery or diagnosis of fetal demise | 32 weeks 6 days | 31 weeks 2 days | 37 weeks 1 day |
| Mode of delivery | Vaginal delivery | Cesarean delivery | Vaginal delivery |
| Pregnancy-related complications | GDMA1 | None | None |
| Prepregnancy BMI, kg/m2 | 22.3 | 32.7 | 23.3 |
| Medications | PNV | PNV, amoxicillin | PNV, protonix |
| TORCH serologies | Toxoplasmosis IgM−/IgG−; parvovirus IgM−/IgG+; CMV IgM−/IgG+; syphilis negative | Not performed | Toxoplasmosis IgM−/IgG−, parvovirus IgM−/IgG+; CMV IgM−/IgG+; syphilis negative |
| Cell-free DNA screening results | Low risk | Low risk | Low risk |
| Neonatal/fetal characteristics | |||
| Birthweight, g | 1395 | 1710 | 2535 |
| Birthweight percentilea | 8 | 73 | 21 |
| Length, cm | NA | 46 | |
| Head circumference, cm | NA | 30 | NA |
| Apgar score at birth | 0,0 | 4,7 | 0,0 |
| Sex | Female | Female | Female |
| Neonate or fetus tested for SARS-CoV-2? | Not tested | Yes, negative | Not tested |
| COVID-19 characteristics | |||
| Gestational week at SARS-CoV-2 infection | 31 | 30 | 35 |
| Severity of SARS-CoV-2 illnessb | Mild | Mild | Mild |
| Symptoms | Fever, cough, body aches, rhinorrhea | Fever, cough, sore throat | Fever, cough, muscle aches |
| Days from initial COVID-19 symptoms to pregnancy outcome | 9 | 5 | 10 |
| COVID-19 vaccination status | Unvaccinated | Unvaccinated | Unvaccinated |
| SARS-CoV-2 plasma viral load, log10 RNA per mL | 1.0c | NA | 1.0c |
| SARS-CoV-2 nasopharyngeal viral load, log10 RNA per mL | NA | 7.4 | 5.5 |
| Variant identified | B.1.617.2 | B.1.617.2 | B.1.617.2 |
Abbreviations: BMI, body mass index; CMV, cytomegalovirus; COVID-19, coronavirus disease 2019; GDMA1, gestational diabetes mellitus class A1; Ig, immunoglobulin; NA, not applicable; PNV, prenatal vitamin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TORCH, toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes virus.
Reference values for birthweight by gestational age at delivery using INTERGROWTH-21st standards.
Severity is defined by National Institutes of Health criteria.
SARS-CoV-2 virus detected below assay limit of quantification. Plasma sample from delivery was not available for analysis in case 2.
Case Demographic and Clinical Characteristics
| Characteristic . | Case 1 . | Case 2 . | Case 3 . |
|---|---|---|---|
| Maternal age, y | 37 | 30 | 39 |
| Gravidity and parity | G4P2012 | G1P0 | G6P5005 |
| Race | Other | White | Black |
| Ethnicity | Hispanic | Non-Hispanic | Non-Hispanic |
| Insurance | Private | Public | Public |
| Pregnancy outcome | Fetal demise | Live birth | Fetal demise |
| Gestational age at delivery or diagnosis of fetal demise | 32 weeks 6 days | 31 weeks 2 days | 37 weeks 1 day |
| Mode of delivery | Vaginal delivery | Cesarean delivery | Vaginal delivery |
| Pregnancy-related complications | GDMA1 | None | None |
| Prepregnancy BMI, kg/m2 | 22.3 | 32.7 | 23.3 |
| Medications | PNV | PNV, amoxicillin | PNV, protonix |
| TORCH serologies | Toxoplasmosis IgM−/IgG−; parvovirus IgM−/IgG+; CMV IgM−/IgG+; syphilis negative | Not performed | Toxoplasmosis IgM−/IgG−, parvovirus IgM−/IgG+; CMV IgM−/IgG+; syphilis negative |
| Cell-free DNA screening results | Low risk | Low risk | Low risk |
| Neonatal/fetal characteristics | |||
| Birthweight, g | 1395 | 1710 | 2535 |
| Birthweight percentilea | 8 | 73 | 21 |
| Length, cm | NA | 46 | |
| Head circumference, cm | NA | 30 | NA |
| Apgar score at birth | 0,0 | 4,7 | 0,0 |
| Sex | Female | Female | Female |
| Neonate or fetus tested for SARS-CoV-2? | Not tested | Yes, negative | Not tested |
| COVID-19 characteristics | |||
| Gestational week at SARS-CoV-2 infection | 31 | 30 | 35 |
| Severity of SARS-CoV-2 illnessb | Mild | Mild | Mild |
| Symptoms | Fever, cough, body aches, rhinorrhea | Fever, cough, sore throat | Fever, cough, muscle aches |
| Days from initial COVID-19 symptoms to pregnancy outcome | 9 | 5 | 10 |
| COVID-19 vaccination status | Unvaccinated | Unvaccinated | Unvaccinated |
| SARS-CoV-2 plasma viral load, log10 RNA per mL | 1.0c | NA | 1.0c |
| SARS-CoV-2 nasopharyngeal viral load, log10 RNA per mL | NA | 7.4 | 5.5 |
| Variant identified | B.1.617.2 | B.1.617.2 | B.1.617.2 |
| Characteristic . | Case 1 . | Case 2 . | Case 3 . |
|---|---|---|---|
| Maternal age, y | 37 | 30 | 39 |
| Gravidity and parity | G4P2012 | G1P0 | G6P5005 |
| Race | Other | White | Black |
| Ethnicity | Hispanic | Non-Hispanic | Non-Hispanic |
| Insurance | Private | Public | Public |
| Pregnancy outcome | Fetal demise | Live birth | Fetal demise |
| Gestational age at delivery or diagnosis of fetal demise | 32 weeks 6 days | 31 weeks 2 days | 37 weeks 1 day |
| Mode of delivery | Vaginal delivery | Cesarean delivery | Vaginal delivery |
| Pregnancy-related complications | GDMA1 | None | None |
| Prepregnancy BMI, kg/m2 | 22.3 | 32.7 | 23.3 |
| Medications | PNV | PNV, amoxicillin | PNV, protonix |
| TORCH serologies | Toxoplasmosis IgM−/IgG−; parvovirus IgM−/IgG+; CMV IgM−/IgG+; syphilis negative | Not performed | Toxoplasmosis IgM−/IgG−, parvovirus IgM−/IgG+; CMV IgM−/IgG+; syphilis negative |
| Cell-free DNA screening results | Low risk | Low risk | Low risk |
| Neonatal/fetal characteristics | |||
| Birthweight, g | 1395 | 1710 | 2535 |
| Birthweight percentilea | 8 | 73 | 21 |
| Length, cm | NA | 46 | |
| Head circumference, cm | NA | 30 | NA |
| Apgar score at birth | 0,0 | 4,7 | 0,0 |
| Sex | Female | Female | Female |
| Neonate or fetus tested for SARS-CoV-2? | Not tested | Yes, negative | Not tested |
| COVID-19 characteristics | |||
| Gestational week at SARS-CoV-2 infection | 31 | 30 | 35 |
| Severity of SARS-CoV-2 illnessb | Mild | Mild | Mild |
| Symptoms | Fever, cough, body aches, rhinorrhea | Fever, cough, sore throat | Fever, cough, muscle aches |
| Days from initial COVID-19 symptoms to pregnancy outcome | 9 | 5 | 10 |
| COVID-19 vaccination status | Unvaccinated | Unvaccinated | Unvaccinated |
| SARS-CoV-2 plasma viral load, log10 RNA per mL | 1.0c | NA | 1.0c |
| SARS-CoV-2 nasopharyngeal viral load, log10 RNA per mL | NA | 7.4 | 5.5 |
| Variant identified | B.1.617.2 | B.1.617.2 | B.1.617.2 |
Abbreviations: BMI, body mass index; CMV, cytomegalovirus; COVID-19, coronavirus disease 2019; GDMA1, gestational diabetes mellitus class A1; Ig, immunoglobulin; NA, not applicable; PNV, prenatal vitamin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TORCH, toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes virus.
Reference values for birthweight by gestational age at delivery using INTERGROWTH-21st standards.
Severity is defined by National Institutes of Health criteria.
SARS-CoV-2 virus detected below assay limit of quantification. Plasma sample from delivery was not available for analysis in case 2.
Of the 2 cases of intrauterine fetal demise (cases 1 and 3), the antenatal courses were uncomplicated. Both patients had negative cell-free DNA screening for common aneuploidies and a normal second trimester detailed anatomic survey ultrasound. Serologies for toxoplasmosis, cytomegalovirus, parvovirus B19, and syphilis were negative for acute infection. No gross anatomic abnormalities were detected at birth in either fetus. Because neither patient with fetal demise consented to fetal autopsy, fetal examinations including SARS-CoV-2 testing were not able to be performed.
In case 2, the patient presented for evaluation 5 days after the onset of COVID-19 symptoms after reporting decreased fetal movement to her outpatient provider. A category 3 fetal heart rate tracing concerning for significant acidemia was identified on fetal heart rate monitoring, which prompted immediate cesarean delivery (emergent). Maternal laboratory evaluation from blood draw on admission was notable for d-dimer level of 49 884 ng/mL, undetectable fibrinogen (<35 mg/dL), and platelets 50 × 103/μL, consistent with disseminated intravascular coagulation (DIC). The cesarean delivery was performed under general anesthesia and was complicated by hemorrhage requiring blood transfusion. In the setting of hemorrhage and transfusion of multiple blood products, the patient remained intubated and was transferred to the intensive care unit for observation. She was extubated on postoperative day 1. She required no therapeutic interventions for COVID-19 disease and her respiratory status remained stable throughout her postpartum recovery, which was uncomplicated. Neonatal Apgar scores at 1 and 5 minutes were 4 and 7, umbilical cord gases were consistent with moderate metabolic acidosis (base excess [BE] < 5th percentile), umbilical artery pH 7.11 (BE −10 mmol/L), and umbilical vein pH 7.15 (BE −8 mmol/L). The initial neonatal course was complicated by respiratory distress syndrome and complications from prematurity consistent with gestational age at delivery of 31 weeks. The neonate tested negative for SARS-CoV-2 by nasopharyngeal RT-PCR on day of life 1, 2, and 15.
Placental Pathology
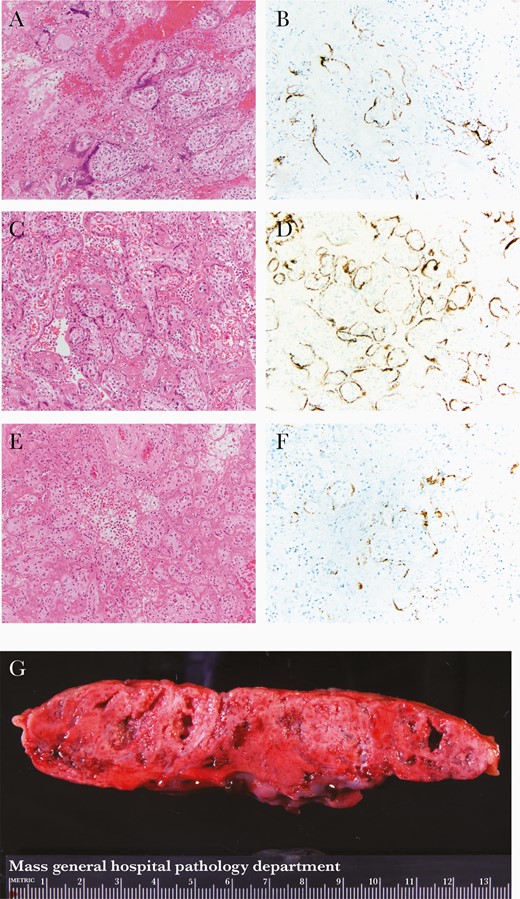
All 3 placentas revealed characteristic gross and microscopic findings of SARS-CoV-2 placentitis [10, 11]: the triad of histiocytic intervillositis, increased perivillous fibrin, and villous trophoblastic necrosis (Figure 1A, 1C, and 1E); a positive SARS-CoV-2 RNA-ISH signal in the villous trophoblast (Figure 1B, 1D, and 1F); and firm and mottled appearing parenchyma grossly (Figure 1G). Other pathological findings present in each case are listed in Supplementary Table 1.
Hematoxylin and eosin (A, C, and E) and SARS-CoV-2 RNA-ISH (B, D, and F) photomicrographs at 20 × original from case 1 (A and B), case 2 (C and D), and case 3 (E and F). A, C, and E, SARS-CoV-2 placentitis: histiocytic intervillositis, increased perivillous fibrin deposition, and villous trophoblast necrosis. B, D, and F, SARS-CoV-2 RNA-ISH showing positive brown particulate signal in a patchy distribution in the villous trophoblast. G, Gross slab section of the parenchyma from case 1 with pale and mottled parenchyma. Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RNA-ISH, RNA in situ hybridization.
SARS-CoV-2 Testing
RNA isolated from biopsies obtained from the maternal and fetal sides of the placenta demonstrated detectable SARS-CoV-2 in all 3 cases (viral load range 3.3 to 6.2 log10 SARS-CoV-2 copies/ng tissue; Supplementary Table 1), with all 3 viral isolates identified as the B.1.617.2 (delta) variant. Maternal plasma samples collected during the delivery hospitalization demonstrated viremia, with SARS-CoV-2 RNA present, but below the assay limit of quantification (< 40 RNA copies/mL, maternal plasma available for case 1 and case 3 only). High nasopharyngeal SARS-CoV-2 viral loads were detected in case 2 and case 3 (7.4 and 5.5 log10 copies RNA/mL, respectively, not available for case 1); sequencing confirmed the delta variant.
DISCUSSION
In this brief report, we present 3 cases, 2 of fetal demise (case 1 and 3) and 1 case of fetal distress necessitating emergent cesarean delivery (case 2), all demonstrating maternal and placental infection with the delta variant and SARS-CoV-2 placentitis [6, 10, 11]. Because neither patient whose pregnancies resulted in fetal demise consented to fetal autopsy, and chromosomal microarray results were not available for 1 patient (case 1) at the time of this report, complete information on an anatomic or genetic cause of death is limited. However, in the setting of low-risk cell free DNA screening for common aneuploidies and a normal anatomical survey ultrasound, the likelihood of a genetic etiology for stillbirth is low. No other potential cause of fetal demise was identified on standard laboratory testing. Our results suggest that the 2 stillbirths were secondary to SARS-CoV-2 placentitis, leading to severe placental damage and insufficiency.
In case 2, the cause of maternal DIC was not identified. Although placental abruption can cause fetal distress and DIC, the lack of vaginal bleeding and/or contractions on presentation is inconsistent with this diagnosis, and no evidence of abruption was identified at delivery or on placental pathology. Although elevated d-dimer and abnormalities of coagulation have been well described in cases of severe COVID-19 and fatal cases of COVID-19 [12], DIC has not yet been identified in the setting of otherwise mild COVID-19 disease. SARS-CoV-2 placentitis certainly may have played a role in the development of fetal distress in case 2, and the fetus may have been vulnerable to placental insults due to small placental size despite normal fetal growth.
With low COVID-19 vaccination rates in pregnant people, recent evidence of risk of greater COVID-19 morbidity in pregnant people infected with variants of concern [8, 9], and evidence that the risk of stillbirth is increased with delta-variant COVID-19 [7], the impact of SARS-CoV-2 variants of concern on pregnancy warrants continued scrutiny. Prior to this report, a definite link between the delta variant, SARS-CoV-2 placentitis, and stillbirth had not been established. Delta variant infections have been linked to higher viral loads and increased risk of hospitalizations compared to prior variants [13]. It is possible that maternal viremia—a feature not commonly observed in pregnant women in the first wave of the pandemic [3], but which was observed in both cases with available blood samples at delivery—can overcome placental immune defenses at the level of the syncytiotrophoblast, which comes into direct contact with maternal blood. Evidence suggests that the delta spike P681R mutation may increase viral infectivity, tissue tropism, and virulence due to enhanced S protein cleavability by furin [14], a transmembrane serine protease that is widely expressed by the syncytiotrophoblast. In the cases presented here, in which placental weights were smaller than expected for gestational age, it is also possible that preexisting placental compromise was worsened by SARS-CoV-2–driven placentitis. We propose SARS-CoV-2 placentitis as a potential mechanistic link between delta-variant SARS-CoV-2 infection during pregnancy and adverse pregnancy outcomes.
Similarities in the 3 cases presented here include third trimester infection, mild COVID-19 disease severity, timing of pregnancy outcome within 14 days of COVID-19 diagnosis, female neonatal sex, small for gestational age placental weight (only in the stillbirth cases), and unvaccinated status. It will be important to elucidate in larger cohorts whether these factors or others predispose to SARS-CoV-2 placentitis and/or stillbirth. Although features of maternal vascular malperfusion, intervillositis, and trophoblastic necrosis have been described in cases of poor pregnancy outcomes and severe or critical maternal COVID-19 disease [15], the same findings have also been reported in cases of mild COVID-19 disease not resulting in fetal demise or compromise [12], and thus maternal disease severity may not correlate well with the risk of adverse fetal outcomes. We suggest that infection with delta variant SARS-CoV-2 during pregnancy, regardless of disease severity, warrants vigilance for signs of fetal compromise in the weeks following SARS-CoV-2 infection, and that antenatal surveillance for placental compromise in the third trimester is warranted. Vaccination against COVID-19 will continue to play a critical role in protecting pregnant people from risks associated with delta-variant COVID-19.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Drs A. Huynh, M. Misialek, V. Torous, and J. Watkins for assistance with the gross and histopathologic diagnoses; the pathologist assistants and histopathologists at the MGH for the gross and histological preparations of the cases; and J. Mendez-Pena for the RNA-ISH studies.
Author contributions. L. L. S., D. J. R., and A. G. E. conceived of the topic and drafted the manuscript. A. G. E. and L. L. S recruited participants and obtained samples. S. B. and J. R. isolated RNA from participant samples. J. Z. L., A. M., and B. E. performed SARS-CoV-2 viral load quantification and sequencing. All authors read and approved of the final manuscript.
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant numbers 1K12HD103096 to L. L. S., 3R01HD100022-02S2 to A. G. E., and UM1AI069412 to J. Z. L.); and March of Dimes (grant number 6-FY20-223 to A. G. E.). A. G. E. is also supported by the Claflin Award from Massachusetts General Hospital Executive Committee on Research.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
J.Z.L., D.J.R., and A.G.E. contributed equally to this work.