-
PDF
- Split View
-
Views
-
Cite
Cite
David E Ausband, Multiple breeding individuals within groups in a social carnivore, Journal of Mammalogy, Volume 99, Issue 4, 13 August 2018, Pages 836–844, https://doi.org/10.1093/jmammal/gyy051
Close - Share Icon Share
Abstract
Breeding strategies of cooperative breeders can vary widely ranging from multiple breeding pairs in a group, to polygamy, polyandry, and combinations of all 3 forms. Often, we do not have a clear understanding of the influences or mechanisms giving rise to the presence of multiple breeding individuals within groups. This is particularly true for animals that are difficult to manipulate or observe, such as large carnivores. I examined factors associated with the occurrence of multiple breeding individuals within groups in a population of recolonizing gray wolves (Canis lupus). Additionally, I investigated what might affect pup recruitment in groups with multiple breeding females. I used population monitoring data for wolves in Idaho and Yellowstone National Park, Wyoming, United States as well as genetic pedigree data for a subset of wolf groups that contained multiple breeding females in Idaho. High wolf density and large group size were both associated with a significant increase in the frequency of multiple breeding females in a group. The probability a pup survived their first year was related positively to the number of breeding females in a group. Multiple breeding can also take the form of polyandry, and “sneaker” males were responsible for paternity in nearly 13% of pups born. Breeding strategies in this social carnivore may be more variable than previously assumed, but their occurrence can be predicted by group size and density. Wolf population projection models and studies regarding reproduction and cooperative breeding in wolves would benefit by incorporating the potential for multiple breeding individuals. Genetic models in particular will be more reliable if they incorporate the potential effect of sneaker males on genetic diversity in a population.
Breeding strategies within groups of cooperative breeders can vary widely. Commonly, groups of cooperative breeders contain a breeding pair and offspring from past reproductive cycles. It is not uncommon, however, for there to be more than 1 breeding pair in a group (Solomon and French 1997). Such multiple breeding can take many forms including multiple breeding pairs in a group, polygamy, polyandry, and combinations of all 3 forms. Additionally, roving individuals unassociated with a group may breed and not remain to rear young, as has been observed in meerkats (Suricata suricatta—Clutton-Brock and Manser 2016). The breeding strategy observed within a population of cooperative breeders can change with resource availability, population density, group size, and intra- and intergroup dynamics (French 1997; Cant et al. 2016; Clutton-Brock and Manser 2016; Koenig et al. 2016). For many species, however, we do not have a clear understanding of the influences or mechanisms giving rise to multiple breeding individuals within groups.
The presence of multiple breeding individuals can have marked and highly variable effects on reproduction within groups. For example, one form of multiple breeding, polygamy, has been shown to manifest as only the litters of the dominant female surviving, some of the litters of subordinate females surviving, or equal survival among all litters (Dietz and Baker 1993; French 1997). One advantage of multiple breeding pairs within groups is an increase in group size and subsequent successful territory defense. Multiple breeding pairs within groups of territorial species also can lead to behaviors such as synchronous parturition to decrease infanticide and potentially increase cooperation and provisioning of all young in a group (Cant et al. 2016). Polyandry may be the least understood breeding strategy, particularly for mammalian cooperative breeders for which detailed genetic data regarding parentage are often lacking. Although polyandry sometimes occurs in mammalian species (Klemme et al. 2008; Lappan 2008), the effects of polyandry on reproduction and what may give rise to its occurrence are not typically known.
Because of its potential effects on group size, breeding by multiple individuals may be particularly influential in groups of social carnivores. In such species, territory defense, successful food acquisition, and ultimately the reproductive output of the group are often linked to group size (Creel and Creel 1995; Stahler et al. 2013; Cassidy et al. 2015). Moreover, territorial social carnivores are often reliant on ephemeral, patchy resources and breeding opportunities within populations are generally limited. Although there may be a dominant breeding strategy arising out of systems where breeding opportunities are limited (i.e., monogamous pair with offspring), we also expect to see a variety of breeding strategies used by individuals to increase their direct and indirect fitness. Long-term manipulative studies of meerkats and banded mongooses (Mungos mungo) provide valuable insights into the variety of breeding strategies used by some cooperatively breeding carnivores (Cant et al. 2016; Clutton-Brock and Manser 2016). Larger social carnivores (e.g., gray wolves; Canis lupus), however, are generally difficult to observe and their populations difficult to manipulate experimentally. Thus, our understanding of breeding strategies used by such species is limited.
In territorial cooperative breeders, opportunities for breeding may become limited as population density increases (Komdeur 1992). In such species, there may be a “polygamy threshold” (Orians 1969) where females choose polygamy rather than accept the high costs of dispersal and low probability of breeding successfully elsewhere. Dominant males may attempt to control all of the breeding opportunities available in a group, but breeding tactics of subordinate males could be affected by population density in a manner similar to the polygamy threshold observed for females. When population density is high and the probability of breeding successfully outside the group is low, a subordinate male may choose to remain in the group and attempt to breed with another subordinate female or through covert copulations with the dominant male’s mate(s), resulting in multiple paternity (Zamudio and Sinervo 2000). Increases in group size in territorial species can be associated with concurrent increases in population density (Watanabe 1981). The frequency of multiple breeding individuals within a group also should increase with group size because there are more opportunities for unrelated individuals to mate (Mech and Boitani 2003) and dominant individuals may have less control over the mating behavior of subordinates (e.g., covert copulations).
While population density and group size may affect the frequency of multiple breeding individuals in a group, both individual and group characteristics can affect how reproductively successful an individual is when there are multiple breeders in a group. For example, young of dominant females may have higher survival than those of subordinates in the group due to dominant females acquiring better resources, provisioning their young better, or simply being older and more experienced at rearing young (Russell et al. 2002). Turnover of breeding males can yield opportunities for breeding by multiple individuals because new breeding males can be unrelated to several females in the group who will willingly mate with them (Mech and Boitani 2003). New breeding males, however, may be unfamiliar with the new territory they occupy and have a difficult time adequately provisioning multiple litters of pups. Large group size, however, may ameliorate such an effect because nonbreeders can help provision and guard young. The effect of group size and helpers on the survival of young may be particularly pronounced in groups of highly related individuals (i.e., due to kin selection—Hamilton 1964; Pope 2000; Griffin and West 2003). The ability of breeders to distinguish their young from those of another may not be strong and multiple breeders in a group may yield increased care and provisioning for all young in the group. Furthermore, for animals whose young move freely about an area and receive food provisions from multiple individuals, competition among young may override any preferential food provisioning by adults. Lastly, breeding females can spend more time guarding young than others in a group (Ausband et al. 2016). Thus, the benefit of having multiple breeding females guarding young in a group likely extends to all young clustered at a rearing site regardless of an individual young’s maternity.
Gray wolves were reintroduced to Idaho and Yellowstone National Park, Wyoming, United States in 1995–1996 (Bangs and Fritts 1996), providing an excellent opportunity to document the occurrence of multiple breeding individuals within groups during a period of recolonization of vacant but suitable habitat. Long-term genetic data collected since 2008 (Ausband et al. 2017a) also provide insights into how various forms of multiple breeding affect recruitment in this large social carnivore. While wolves are thought to be generally monogamous, polygyny has been documented in the wild (Mech and Nelson 1989; Borg et al. 2014), thus the behavioral flexibility exists for alternative breeding strategies. Although breeding by multiple individuals within groups has been recorded, the rate of breeding failure in these cases was thought to be quite high, resulting in survival of young from just 1 breeding pair per group (Harrington et al. 1982; Packard et al. 1983). There is evidence of subordinate female wolves breeding in their natal group in addition to the group’s traditional breeding pair (Mech and Boitani 2003). Such multiple breeding is thought to be by mothers and their daughters with dominant males or possibly unrelated males via temporary affiliations (Mech and Boitani 2003), although genetic data to support these hypotheses are generally lacking.
I investigated what factors were associated with the occurrence of multiple breeding females in groups in a population of recolonizing gray wolves. Specifically, I predicted that the probability of multiple breeding females in groups would increase with 1) wolf population density, and 2) wolf group size. Breeding by multiple individuals in groups also may depend on other factors, such as food abundance (Fuller et al. 2003; Clutton-Brock and Manser 2016). To examine the potential influence of prey density on the occurrence of multiple individuals breeding, I also performed a subset analysis that included elk (Cervus canadensis) density as a predictor variable where such data were available.
Additionally, I investigated what might affect pup recruitment in groups when there were multiple breeding females. I predicted that pup recruitment in groups containing multiple breeding females would be positively influenced by 1) maternal dominance status, 2) maternal breeding tenure (i.e., years breeding), 3) lack of turnover in breeding males, 4) group size (i.e., number of adults), 5) number of nonbreeding helpers, 6) genetic relatedness between the breeding female and remaining adults in the group, and 7) number of breeding females in the group. While the aforementioned analyses focus on multiple breeding females, various mating strategies might be used by both sexes in a population. Thus, I used detailed pedigree data from genetically sampled wolf groups in Idaho to document the suite of mating strategies used in a free-ranging population of wolves.
Methods and Materials
Study areas
To assess what factors were associated with the occurrence of multiple breeding females in a group, I used data from wolves monitored after wolf reintroduction to Idaho and Yellowstone National Park, Wyoming, United States. Wolves were generally not harvested in the study areas during the time period I analyzed (1996–2002 in Idaho and 1996–2012 in Yellowstone National Park). Public harvest began in states surrounding Yellowstone National Park in 2009 and 18 wolves residing in packs in the park during summer were harvested outside of the park during hunting seasons from 2009 to 2012. Yellowstone National Park (8,983 km2) is dominated by lodgepole pine (Pinus contorta) forests and expansive meadow systems. The park is relatively dry and precipitation averages 47 cm annually; temperature fluctuations range from −39°C in winter to 30°C in summer at Yellowstone Lake (Western Regional Climate Center 2014). There is no hunting by humans and elk are the primary prey of wolves in the park.
To assess what effects multiple breeding females in groups may have on pup recruitment, I used data from 3 genetically censused wolf groups (6 wolf-group-years) in Game Management Unit (GMU) 28 (3,388 km2), in Idaho, 2008–2011. I also used data from genetically censused wolf groups in GMUs 28 and 33–35 (3,861 km2) in Idaho during 2008–2016 to summarize the different types of multiple breeding documented. Idaho is mountainous and dominated by a mix of ponderosa pine (P. ponderosa), lodgepole pine, and spruce (Picea englemannii) forests and sagebrush (Artemisia tridentata) steppe. Annual precipitation ranges from 89 to 178 cm and temperatures range from −34°C in winter to 38°C in summer (Western Regional Climate Center 2014). Elk were the primary prey for wolves during the time period we analyzed in Idaho and public harvest of wolves did not begin until 2009 (Ausband 2016).
Occurrence of multiple breeding females in a group
To assess what factors were associated with the occurrence of multiple breeding females in a group, I used data derived from tracking radiocollared individuals and observing groups from fixed-wing aircraft and ground surveys at wolf reproductive sites (i.e., dens, rendezvous sites) in Idaho and Yellowstone National Park. Data were collected as part of annual requirements under Endangered Species Act regulations. I tabulated wolf density (wolves/1,000 km2), the number of breeding females in a group, and group size for wolf groups and populations (Phillips and Smith 1997; Mack and Laudon 1998; Smith et al. 1999, 2000, 2003, 2004, 2005, 2006, 2007, 2008, 2009, 2010, 2011, 2012, 2013; Mack et al. 2002; Smith and Guernsey 2002; Mack and Holyan 2003). For these analyses (in contrast to genetic analyses described below), the presence of multiple breeding males was not known because these data were observational. Breeding females, however, commonly occupy separate dens until pups are weaned and thus multiple breeding females can be tallied within groups.
I assessed the influence of density and group size on the probability a group contained multiple breeding females using a mixed effects model and a binomial distribution with a logit link function. I included random effects for study area, year, and group in all models and compared candidate models using Akaike’s Information Criterion (AIC—Burnham and Anderson 2002). I considered a covariate influential in the most-supported model when P < 0.05.
For a subset of wolf groups and years for which such data were available, I also assessed the influence of the previous winters’ elk density (elk/km2) using data from aerial counts in winter (NYCWWG 2012), wolf population density, and group size on the probability of multiple breeders in wolf groups in the northern range of Yellowstone National Park, 1996–2010. I used a logistic regression mixed effects model with a binomial distribution, logit link function, and included random effects for year and group. I compared candidate models using AIC (Burnham and Anderson 2002).
Influence of multiple breeding females on pup recruitment
To assess the effects of multiple breeding females in groups on pup recruitment, field crews collected scats for genetic analysis at rendezvous sites of reproductively active wolf groups. When available, I used radiotelemetry locations of wolves to locate rendezvous sites. In areas that did not contain radiocollared wolves, field crews surveyed at historic and highly suitable (≥ 70%) rendezvous sites predicted by a habitat model (Ausband et al. 2010). Technicians typically gathered 125–200 samples per group per year and attempted to locate and resample each group every year. DNA was extracted from scats at the University of Idaho Laboratory for Ecological, Evolutionary and Conservation Genetics and amplified at 18 microsatellite loci to determine individual identification. Detailed field and genetic analysis protocols can be found in Ausband et al. (2010), Stenglein et al. (2010a, 2010b), Stenglein et al. (2011), and Stansbury et al (2014). Protocols and field sampling aligned with guidelines of the American Society of Mammalogists (Sikes et al. 2016).
I had a subsample of groups where both multiple breeding females were present and genetic data were available for each individual in the group. I used these data to construct pedigrees via maximum likelihood in Program COLONY version 2.0.5.5 (Jones and Wang 2009) and assess the influence of covariates on the probability of pup survival to 15 months of age (approximately 15 Julyt–15 Julyt+1). For each year, I included all genetically sampled adult males and females as potential parents and all sampled pups as potential offspring and then determined breeders and their offspring from resulting pedigrees. As part of state population monitoring efforts, wolves were sampled genetically in various areas across the state and tissue samples were collected from radiocollared and harvested wolves as well. Resulting adult genotypes were included in analyses as potential breeders. I calculated allele frequencies for each year in Program COANCESTRY version 1.0.1.5 (Wang 2011) and then imported those values into Program COLONY for use in pedigree analyses. I allowed for polygamy in both males and females and assumed an allelic dropout rate of 0.01. In cases where parentage was undetermined from COLONY, I further examined offspring genotypes against the likely parents of the remaining offspring in the group and allowed for a two-allele mismatch owing to allelic dropout between parent and offspring to verify parentage across the 18 loci using exclusion methods (Allendorf et al. 2013). I estimated the average genetic relatedness between the breeding female and other adult wolves in the group using triadic likelihood estimates from Program COANCESTRY (Wang 2011).
I assessed the influence of covariates affecting the probability of pup recruitment in a subsample of groups with multiple breeding females using mixed effects models with a binomial distribution and a logit link function. I included random effects for breeding female identification number, group, and year in each model. I used the “lme4” and “glmm” packages in Program R (version 3.3.0—The R Foundation for Statistical Computing 2016) for analyses. I compared candidate models using AIC (Burnham and Anderson 2002). I considered a covariate influential in the most-supported model when P < 0.05.
Results
Wolf population density varied widely from 0.57 wolves/1,000 km2 in Idaho after reintroduction in 1996 to a high of 54.9 wolves/1,000 km2 on Yellowstone National Park’s northern range in 2008. Average wolf density for 221 wolf-group-years analyzed was 16.2 wolves/1,000 km2 (SE = 1.1). Group size ranged from 1 to a high of 26 adults in a group on Yellowstone National Park’s northern range in 2001. Overall, group size averaged 6.7 adults/group (SE = 0.29). Multiple breeding females were documented in 15.8% of wolf groups totaling 78 breeding females (20% of groups had > 2 breeding females). Data can be found in Supplementary Data SD1.
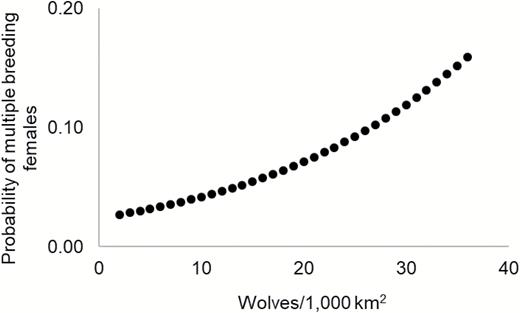
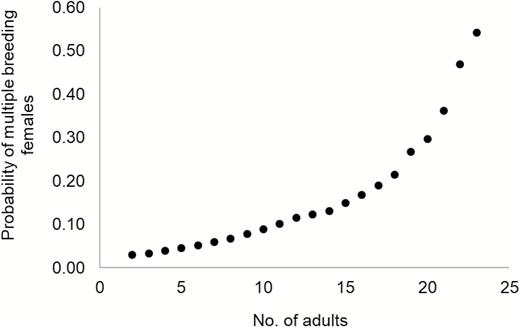
Population density and number of adults per group were not strongly correlated (Pearson’s product moment correlation = 0.33) thus both variables were retained in models. Models populated with data from 221 wolf-group-years (groups = 67, years = 17) predicted that increasing wolf density was associated with a significant increase (β = 0.06; P = 0.003) in the probability of multiple breeding females in groups (Fig. 1). The odds a group contained > 1 breeding female increased 5.8% (odds ratio 95% CI = 1.9–9.8%) for every 1-unit increase in wolf density given other covariates remained constant. Increasing group size (number of adults) was also associated with an increase (β = 0.15; P = 0.02) in the probability of multiple breeding females in groups (Fig. 2). The odds a group contained > 1 breeding female increased 15.8% (odds ratio 95% CI = 2.0–31.6%) for every 1-unit increase in group size given other covariates remained constant. The most-supported model for predicting multiple breeding females in a group contained both population density and group size variables (Table 1). The SD of the random effects for group and year indicated they varied considerably relative to the fixed effects of group size and density (Group SD = 0.96, Year SD = 0.62, Area SD = 0.00). Attempts to fit models with population density and number of adults per group as an interaction term failed to converge.
The probability of multiple breeding females in groups as a function of wolf population density when group size is held constant (n = 6 adults), Idaho and Yellowstone National Park, Wyoming, United States, 1996–2012.
The probability of multiple breeding females in groups as a function of wolf group size (number of adults) when wolf density is held constant (15 wolves/1,000 km2), Idaho and Yellowstone National Park, Wyoming, United States, 1996–2012.
Comparison of mixed effects logistic regression models predicting the probability of multiple breeding females as a function of population density (wolves/1,000 km2) and group size (number of adults), Idaho and Yellowstone National Park, Wyoming, 1996–2012, n = 221 observations. ΔAIC = difference between model AIC and lowest AIC in the model set; w = Akaike model weight; k = number of parameters; −2LL = −2 * log-likelihood.
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| Group size + wolf density | 0 | 0.81 | 6 | 149.0 |
| Wolf density | 3.3 | 0.15 | 5 | 154.4 |
| Group size | 6.0 | 0.04 | 5 | 157.0 |
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| Group size + wolf density | 0 | 0.81 | 6 | 149.0 |
| Wolf density | 3.3 | 0.15 | 5 | 154.4 |
| Group size | 6.0 | 0.04 | 5 | 157.0 |
Comparison of mixed effects logistic regression models predicting the probability of multiple breeding females as a function of population density (wolves/1,000 km2) and group size (number of adults), Idaho and Yellowstone National Park, Wyoming, 1996–2012, n = 221 observations. ΔAIC = difference between model AIC and lowest AIC in the model set; w = Akaike model weight; k = number of parameters; −2LL = −2 * log-likelihood.
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| Group size + wolf density | 0 | 0.81 | 6 | 149.0 |
| Wolf density | 3.3 | 0.15 | 5 | 154.4 |
| Group size | 6.0 | 0.04 | 5 | 157.0 |
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| Group size + wolf density | 0 | 0.81 | 6 | 149.0 |
| Wolf density | 3.3 | 0.15 | 5 | 154.4 |
| Group size | 6.0 | 0.04 | 5 | 157.0 |
Subset analyses of models populated with data from 85 wolf-group-years (groups = 25, years = 15) showed little support for the influence of elk density on the probability a group contained multiple breeding females (Table 2). A model with elk density performed no better than a null model (intercept + random effects). The included random effect of group accounted for little variation in the model, but year varied considerably relative to the fixed effects (Group SD = 0.00, Year SD = 0.51; group size β = 0.22).
Results from subset analyses showing AIC values from mixed effects logistic regression models predicting the probability of multiple breeding females as a function of wolf population density (wolves/1,000 km2), group size (number of adults), and previous winters’ elk density (elk/km2) on Yellowstone National Park’s northern range, Wyoming, 1996–2010, n = 85 observations. “Null” model is an intercept and random effects only model. ΔAIC = difference between model AIC and lowest AIC in the model set; w = Akaike model weight; k = number of parameters; −2LL = −2 * log-likelihood.
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| Group size | 0 | 0.50 | 4 | 97.6 |
| Group size + wolf density | 1.2 | 0.27 | 5 | 96.8 |
| Global | 1.6 | 0.22 | 6 | 95.2 |
| Null | 9.9 | 0.00 | 3 | 109.6 |
| Elk density | 11.0 | 0.00 | 4 | 108.8 |
| Wolf density | 11.7 | 0.00 | 4 | 109.4 |
| Wolf density + elk density | 12.9 | 0.00 | 5 | 108.6 |
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| Group size | 0 | 0.50 | 4 | 97.6 |
| Group size + wolf density | 1.2 | 0.27 | 5 | 96.8 |
| Global | 1.6 | 0.22 | 6 | 95.2 |
| Null | 9.9 | 0.00 | 3 | 109.6 |
| Elk density | 11.0 | 0.00 | 4 | 108.8 |
| Wolf density | 11.7 | 0.00 | 4 | 109.4 |
| Wolf density + elk density | 12.9 | 0.00 | 5 | 108.6 |
Results from subset analyses showing AIC values from mixed effects logistic regression models predicting the probability of multiple breeding females as a function of wolf population density (wolves/1,000 km2), group size (number of adults), and previous winters’ elk density (elk/km2) on Yellowstone National Park’s northern range, Wyoming, 1996–2010, n = 85 observations. “Null” model is an intercept and random effects only model. ΔAIC = difference between model AIC and lowest AIC in the model set; w = Akaike model weight; k = number of parameters; −2LL = −2 * log-likelihood.
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| Group size | 0 | 0.50 | 4 | 97.6 |
| Group size + wolf density | 1.2 | 0.27 | 5 | 96.8 |
| Global | 1.6 | 0.22 | 6 | 95.2 |
| Null | 9.9 | 0.00 | 3 | 109.6 |
| Elk density | 11.0 | 0.00 | 4 | 108.8 |
| Wolf density | 11.7 | 0.00 | 4 | 109.4 |
| Wolf density + elk density | 12.9 | 0.00 | 5 | 108.6 |
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| Group size | 0 | 0.50 | 4 | 97.6 |
| Group size + wolf density | 1.2 | 0.27 | 5 | 96.8 |
| Global | 1.6 | 0.22 | 6 | 95.2 |
| Null | 9.9 | 0.00 | 3 | 109.6 |
| Elk density | 11.0 | 0.00 | 4 | 108.8 |
| Wolf density | 11.7 | 0.00 | 4 | 109.4 |
| Wolf density + elk density | 12.9 | 0.00 | 5 | 108.6 |
I predicted the probability of survival to 15 months for 45 pups from 9 breeding females and 3 groups. The only variable that was significantly associated with the probability of a pup surviving to 15 months was the number of breeding females in a group; survival of pups was greater when there was a greater number of breeding females (β = 1.65; P = 0.03; Table 3). The included random effects did not account for substantial variation in the model (all random effects = 0.00). Subset analyses yielded no significant influence of breeding female tenure (number of years breeding position held) on pup survival for dominant (P = 1.0) or subordinate (P = 0.85) females.
Comparison of mixed effects logistic regression models predicting the probability of recruitment (survival to 15 months) for pups in groups of wolves with multiple breeding females, Idaho 2008–2011, n = 45 observations. BFs = breeding females; NBFs = nonbreeding females; NBMs = nonbreeding males; ΔAIC = difference between model AIC and lowest AIC in the model set; w = Akaike model weight; k = number of parameters; −2LL = −2 * log-likelihood.
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| No. of BFs | 0 | 0.55 | 5 | 51.6 |
| Genetic relatedness | 3.1 | 0.12 | 5 | 54.6 |
| No. of NBFs | 3.9 | 0.08 | 5 | 55.4 |
| No. of adults | 4.0 | 0.07 | 5 | 55.6 |
| New breeding male | 4.1 | 0.07 | 5 | 55.6 |
| No. of NBMs | 4.3 | 0.06 | 5 | 55.8 |
| Dominant female | 4.7 | 0.05 | 5 | 56.2 |
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| No. of BFs | 0 | 0.55 | 5 | 51.6 |
| Genetic relatedness | 3.1 | 0.12 | 5 | 54.6 |
| No. of NBFs | 3.9 | 0.08 | 5 | 55.4 |
| No. of adults | 4.0 | 0.07 | 5 | 55.6 |
| New breeding male | 4.1 | 0.07 | 5 | 55.6 |
| No. of NBMs | 4.3 | 0.06 | 5 | 55.8 |
| Dominant female | 4.7 | 0.05 | 5 | 56.2 |
Comparison of mixed effects logistic regression models predicting the probability of recruitment (survival to 15 months) for pups in groups of wolves with multiple breeding females, Idaho 2008–2011, n = 45 observations. BFs = breeding females; NBFs = nonbreeding females; NBMs = nonbreeding males; ΔAIC = difference between model AIC and lowest AIC in the model set; w = Akaike model weight; k = number of parameters; −2LL = −2 * log-likelihood.
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| No. of BFs | 0 | 0.55 | 5 | 51.6 |
| Genetic relatedness | 3.1 | 0.12 | 5 | 54.6 |
| No. of NBFs | 3.9 | 0.08 | 5 | 55.4 |
| No. of adults | 4.0 | 0.07 | 5 | 55.6 |
| New breeding male | 4.1 | 0.07 | 5 | 55.6 |
| No. of NBMs | 4.3 | 0.06 | 5 | 55.8 |
| Dominant female | 4.7 | 0.05 | 5 | 56.2 |
| Model . | ΔAIC . | w . | k . | −2LL . |
|---|---|---|---|---|
| No. of BFs | 0 | 0.55 | 5 | 51.6 |
| Genetic relatedness | 3.1 | 0.12 | 5 | 54.6 |
| No. of NBFs | 3.9 | 0.08 | 5 | 55.4 |
| No. of adults | 4.0 | 0.07 | 5 | 55.6 |
| New breeding male | 4.1 | 0.07 | 5 | 55.6 |
| No. of NBMs | 4.3 | 0.06 | 5 | 55.8 |
| Dominant female | 4.7 | 0.05 | 5 | 56.2 |
Most genetically sampled packs (71.9%) were comprised of a single breeding pair; however, I documented a wide variety of breeding tactics used by wolves ranging from polygamy to polyandry. Examples include: polygamy among group members (n = 2; 3.1%), distinct multiple breeding pairs within a group (n = 1; 1.6%), distinct multiple breeding pairs within a group plus breeding with individuals from outside the group (n = 3; 4.7%), polyandry among group members (n = 1; 1.6%), polyandry among group members plus breeding with individuals from outside the group (n = 3; 4.7%), sneaker male unaffiliated with group and polygamy (n = 1; 1.6%). Sneaker males (i.e., generally males unaffiliated with the group) were responsible for shared paternity in nearly 13% of wolf groups sampled (n = 8) and 34 of 266 (12.8%) pups born.
Discussion
It was not uncommon for groups of this social carnivore to contain multiple breeding females at high population densities or when group size was large. Ultimately, the probability a pup survived their first year was strongly and positively related to the number of breeding females in the group. Multiple breeding individuals also took the form of polyandry, and sneaker males contributed to paternity in many groups (nearly 13% of pups sampled genetically). Mating systems are a function of individuals, not evolutionarily defined for a species (Clutton-Brock 1989), and the variety of mating strategies I observed in gray wolves supports this. Many mating systems are viewed as the outcome of different degrees of mate guarding, ultimately dependent on various environmental factors (Clutton-Brock 1989). Generally, I did not know if 1 male bred with multiple females or ≥ 1 male bred with several females in groups where multiple breeding females occurred. The majority of genetically sampled wolf groups, however, showed a single breeding pair and most cases of multiple breeding individuals involved multiple breeding males, suggesting mate guarding is not always successful in wolves. This is further illustrated by the preponderance of sneaker males and their genetic contributions in my study populations in Idaho. For gray wolves, and perhaps species with similar life histories, monogamy may be the expected mating system at low density and alternative mating strategies emerge as density increases.
The occurrence of multiple breeding individuals varied considerably by group and year, but density was a strong predictor of the prevalence of multiple breeding females. Such a finding suggests that breeding opportunities are limited in this species and when they are, females may resort to polygamy. Polygamy thresholds as a function of population density have been documented in cooperatively breeding tamarins (Saguinus fuscicollis—Goldizen and Terborgh 1989), although I do not know of another study that has shown this in a large social carnivore. Choosing to remain in groups and share breeding opportunities when density is high implies the habitat is saturated. Wolves can increase fitness even when density is high through avenues other than breeding in their group, however. For example, an individual can gain indirect fitness benefits by rearing siblings, and forays to nearby groups and covert copulations can greatly increase an individual’s fitness even when habitat is saturated and traditional breeding opportunities are limited. I documented several instances of such covert breeding, indicating that it may be a fairly common breeding tactic in gray wolves.
Generally, only larger groups (> 8 adults) showed evidence of multiple breeding females. Similar effects of group size on multiple breeding individuals have been documented in golden lion tamarins (Leontopithecus rosalia—Dietz and Baker 1993) but not in other large social carnivores to my knowledge. For example, the occurrence of multiple breeding individuals in groups of gray wolves in Alaska was not correlated with group size (Ballard et al. 1987) but inference was drawn from just 3 of 41 wolf groups. Group size likely influences the opportunity for unrelated animals to breed either through direct adoption into the group or through temporary affiliations with individuals from outside the group. Such affiliations may be difficult for dominant breeders to deter behaviorally when group size is large and individual group members are temporarily separated from the larger group. For large carnivores that generally subsist on widely scattered prey, very large group size may be disadvantageous for all group members (Creel and Creel 1995) and multiple breeding individuals would only exacerbate this. It is reasonable to assume that a threshold exists where group size and its subsequent effect on multiple breeding individuals has deleterious effects on survival, but my genetic analyses failed to yield such evidence, at least for young of the year.
Subset analyses did not show an effect of elk density on the occurrence of multiple breeding individuals within groups of wolves in Yellowstone National Park. Carnivore population demography, and reproduction in particular, has been linked to food availability in many ecological systems (Fuller et al. 2003; Mcroberts and Mech 2014; Clutton-Brock and Manser 2016). Breeding by multiple individuals, except possibly by sneaker males, should be contingent on adequate food resources being available. When food resources are low, individuals may attempt to breed, but poor body condition in females could inhibit successful pregnancy. Additionally, young may be born but die because of malnourishment. Unless a female attempted to den, we did not know if some were pregnant but failed to ultimately produce pups. Despite declines on Yellowstone’s northern range over the time period examined in the subset analyses, elk density was still relatively high compared to the park’s interior and much of Idaho. Assuming food abundance was adequate, density dependent factors such as group size and wolf density were better predictors of multiple breeding individuals.
Only one of my hypotheses regarding recruitment in groups with multiple breeding females was supported; the number of breeding females in a group was positively associated with pup survival. Previous work (Harrington et al. 1982; Packard et al. 1983) suggested that pups from just a single female survived when there were instances of multiple breeding individuals. My results did not support these findings. The number of breeding females in a group also had a strong effect on the survival of young tamarins (Dietz and Baker 1993). I note that the sample size for groups with both multiple breeding females and pup survival data is small, just 45 pups from 9 breeding females. Thus, the association between the number of breeding females and increased pup survival may be an artefact of small sample size. The lack of an effect of other variables I considered (e.g., dominant female tenure) may likewise be due to lack of statistical power. If the positive association between number of breeding females and pup survival is true, this could occur by several means. Breeding female wolves in a group generally give birth to young in separate dens but commonly join their pups with young of other females in the group once pups are weaned (Smith et al. 2010). Breeding females may be unable to discern their young from those of another female at post-weaning pup-rearing sites and as a consequence may inadvertently provision another female’s young. Competition among pups also may yield benefits for some pups that are not a female’s progeny particularly during meat regurgitation events, which can be behavioral melees at pup-rearing sites. Lastly, breeding females spend more time guarding pups at pup-rearing sites than other group members (Ausband et al. 2016) and pups may benefit from this behavior regardless of maternity.
I documented an unexpectedly high percentage of groups (12.5%) with at least some young sired by males unaffiliated with the group. The presence of sneaker males and the usefulness of this breeding tactic are likely affected by population density as well, but I did not have sufficient data to test such a hypothesis. Such sneaker males may be residents of adjacent unsampled groups or loner wolves existing in the interstitial space between group territories (Mech and Boitani 2003). Extra-group paternity has been documented in other cooperatively breeding species such as meerkats (Clutton-Brock and Manser 2016). In meerkats, however, sneaker males typically breed with subordinate females or females in groups lacking an unrelated male with which to breed (Clutton-Brock and Manser 2016). In gray wolves, I found similar examples of such behavior but also documented sneaker males breeding with dominant females in intact, unrelated breeding pairs. The frequency of sneaker males and their subsequent paternity in my sample raises the question about their potential effect on genetic diversity in a population of cooperative breeders. For example, projections of genetic diversity in a population of reintroduced wolves estimated significant inbreeding depression in the population over the long-term using samples collected at the time of the study (Vonholdt et al. 2008), although see Vonholdt et al. (2010) for revised results using additional data. The projections presented, however, did not allow for multiple breeding individuals within groups or shared paternity, which were detected in 30% and 13% of my sample, respectively. Such data were not available at the time of the Vonholdt et al. (2008) study, but breeding strategies of wolves may be more variable than previously assumed and this variation should be incorporated into future models of wolf population genetics.
Sneaker males and their reproductive strategy are expected to increase in frequency with population density, but I found no evidence for this. Although the sample size was limited to 8 events, 4 sneaker male events occurred before harvest began in Idaho. After harvest began, group size and density declined in the population (Ausband et al. 2017b), yet 4 sneaker male events occurred in subsequent years. Current evidence, although limited, suggests sneaker males are present across a range of population densities and they contribute genetically within populations.
Some subordinate females that breed may lose their young early in the pre-weaning period because of a lack of help or infanticide (Peterson et al. 1984; Smith et al. 2015). Such losses would have gone undetected in my genetic sampling because I sampled wolf groups at post-weaning pup-rearing sites (approximately mid-July). That I found no difference in dominance status on the survival of pups applies only to females whose pups survived long enough to make it to post-weaning pup-rearing sites (generally pups 3 months old).
Supplementary Data
Supplementary data are available at Journal of Mammalogy online.
Supplementary Data SD1.—Data used to test for effects of group size and population density on the probability of multiple breeding in gray wolves in Idaho (1996–2002) and Yellowstone Park, United States (1996–2012).
Acknowledgments
I used data collected in the northern Rocky Mountains of the United States since wolf reintroduction began in 1995. Many agency personnel and members of the public were involved in data collection and I thank them for their efforts and time. Numerous dedicated technicians worked hard to collect genetic data and I sincerely thank them for their incredible efforts in the field. I also thank H. Cooley, M. Hurley, J. Husseman, C. Mack, T. Martin, M. Mitchell, M. Lucid, J. Rachael, S. Nadeau, S. Roberts, D. Smith, D. Stahler, C. Stansbury, J. Stenglein, J. Struthers, P. Zager, and Lisette Waits’ Laboratory for Ecological, Evolutionary and Conservation Genetics at the University of Idaho for their assistance. I was supported by Idaho Department of Fish and Game while writing this manuscript. Additionally, for genetic sampling and analyses, I received funding from the Coypu Foundation, Regina Bauer Frankenberg Foundation for Animal Welfare, Bernice Barbour Foundation, Wesley M. Dixon Fellowship at the University of Montana, Idaho Department of Fish and Game, Eppley Foundation for Scientific Research, Leonard X. Bosack and Bette M. Kruger Foundation, Nez Perce Tribe, Oregon Zoo Future for Wildlife grants, Shikar Safari Club International, Steven Leuthold Family Foundation, the Mountaineers Foundation, U.S. Fish and Wildlife Service, Wilburforce Foundation, Wolf Recovery Foundation, and the University of Idaho Environmental Science Program.
Literature Cited
R Foundation for Statistical Computing. 2016. R Core Team. R: A language and environment for statistical computing.