-
PDF
- Split View
-
Views
-
Cite
Cite
Xi Wan, Jan Hontelez, Allessandra Lillo, Chiara Guarnerio, Diederik van de Peut, Elena Fedorova, Ton Bisseling, Henk Franssen, Medicago truncatula ENOD40-1 and ENOD40-2 are both involved in nodule initiation and bacteroid development, Journal of Experimental Botany, Volume 58, Issue 8, June 2007, Pages 2033–2041, https://doi.org/10.1093/jxb/erm072
Close - Share Icon Share
Abstract
The establishment of a nitrogen-fixing root nodule on legumes requires the induction of mitotic activity of cortical cells leading to the formation of the nodule primordium and the infection process by which the bacteria enter this primordium. Several genes are up-regulated during these processes, among them ENOD40. Here it is shown, by using gene-specific knock-down of the two Medicago truncatula ENOD40 genes, that both genes are involved in nodule initiation. Further, during nodule development, both genes are essential for bacteroid development.
Introduction
Root nodules are specialized organs on the roots of legumes in which soil-borne bacteria, collectively known as rhizobia, are hosted intracellularly and fix atmospheric nitrogen. The formation of these organs requires a complex communication between the bacteria and their host plants. Plant-secreted flavonoids are inducers of bacterial genes that code for proteins involved in the production of so-called Nod factors. These molecules are lipo-chito-oligosaccharides consisting of a skeleton of 4,5 N-acetyl glucosamines, substituted with specific modifications (Spaink, 2000). Nod factors are recognized by plant receptors that activate a Nod factor signalling pathway. This induces mitotic activity in already differentiated cortical cells. These dividing cortical cells form the nodule primordium. Meanwhile, bacteria enter the root hair through a tube-like structure, the so-called infection thread. These threads grow towards the primordium and, upon arrival, bacteria are released from the threads. The bacteria become entrapped within the plant plasma membrane and form the so-called symbiosomes. After infection, the nodule primordium differentiates into a nodule (Stougaard, 2001; Limpens and Bisseling, 2003). Medicago truncatula nodules have a persistent meristem at their apex, and nodule cells are of graded age along the apical–basal axis. Therefore, based on both plant and rhizobial cell morphology (Vasse et al., 1990; Patriarca et al., 2004) and gene expression (Scheres et al., 1990), indeterminate nodules can be divided into four distinct zones, while five stages of bacterial development can be distinguished (Vasse et al., 1990; Patriarca et al., 2004). At the distal end, the meristem forms zone I. Cells of the meristem are small and rich in cytoplasm, while infection threads and bacteria are absent. Infection threads enter the cells at the distal end of zone II, the infection zone. Here, rhizobia are released from the infection threads and are surrounded by a plant membrane, together forming the symbiosome (rhizobia in stage I of development). The symbiosomes divide and the short rod-like rhizobia start to elongate (stage II). Rhizobia from this stage on are named bacteroids (Bergersen, 1974). In cells in the proximal part of the infection zone, bacteroids stop elongating and have a long rod-shaped structure (stage III). In cells of the fixation zone, zone III, the infected cells are fully packed with elongated bacteroids and the vacuoles of the cells have almost completely disappeared. At the distal part of the fixation zone, stage IV bacteroids are morphologically more heterogeneous and capable of fixing nitrogen. In older nodules, at the base of the nodule a zone can be distinguished where bacteroids disintegrate (stage V) and plant cells begin senescence. This zone is called the senescence zone (zone IV).
During nodulation, several host genes are up-regulated, indicating that these genes are important for establishing a symbiosis between the plant and rhizobia. ENOD40 is one of these genes (Kouchi and Hata, 1993; Yang et al., 1993; Crespi et al., 1994) and its expression level is increased at the onset of nodulation (Compaan et al., 2001). ENOD40 is first expressed in pericycle cells and later in nodule primordium cells. Later in symbiosis ENOD40 is expressed in cells of the nodule where differentiation of host cells and rhizobia is initiated.
ENOD40 has an unusual structure, since it lacks a long open reading frame (ORF). However, several short ORFs are present (Sousa et al., 2001) in ENOD40 transcripts. Therefore, it is possible that these oligopeptides are translated and that a peptide represents the biological activity of ENOD40. Alternatively, due to the lack of a long ORF and the highly structured RNA (Girard et al., 2003), it has been suggested that the ENOD40 activity resides in the RNA (Crespi et al., 1994; Sousa et al., 2001; Girard et al., 2003). At the nucleotide level, ENOD40 transcripts share two regions of high sequence similarity, named box1 and box2 (Kouchi et al., 1999). Some of the short ORFs reside in these regions. In particular, a 10–13 amino acid oligopeptide encoded by the ORF in box1 is conserved among plant species (Compaan et al., 2001; Varkonyi-Gasic and White, 2002), with the exception of Casuarina glutinosa (Santi et al., 2003). The high degree of conservation of box1 and box2 sequences indicates that these regions are important for ENOD40 function. However, it remains to be solved whether the ENOD40 acitivity is peptide or RNA mediated.
The spatial and temporal expression of ENOD40 suggests that this gene could play an important role in nodule development. Recently, it was reported that knock-down of Lotus japonicus ENOD40 (LjENOD40) expression by RNA interference (RNAi) leads to a strong reduction in nodule number, but bacterial infection of root hairs was not affected (Kumagai et al., 2006). Also, in M. truncatula, ENOD40 might be important in nodule initiation as in plants in which ENOD40 expression is down-regulated due to co-suppression (Charon et al., 1999) nodule number is markedly reduced. Although these studies indicate that ENOD40 has an important role in nodule initiation, none of the forward genetic screens for legume mutants disturbed in nodule formation has resulted in an ENOD40 mutant. This might be due to functional redundancy or, alternatively, it indicates that ENOD40 is not essential for nodule formation.
Several legumes, such as Lotus (Flemetakis et al., 2000) and Trifolium repens (Varkonyi-Gasic and White, 2002), have more than one copy of ENOD40. However, to date, only one ENOD40 gene has been identified in M. truncatula. Recently, an expressed sequence tag (EST) has been deposited in the M. truncatula database, the nucleotide sequence of which shows homology to MtENOD40. The identification of a putative second MtENOD40 gene, MtENOD40-2, in M. truncatula opens up the possibility of testing whether both genes are required for nodule initiation and development.
To this end, gene-specific RNAi was applied to knock-down the expression of both genes separately in M. truncatula roots.
Materials and methods
Primers
The folowing primers were used: p402Hind, GGAAGCTTATCCTTAAGCTAAAAAAAAATCAGG; p402Bam, GGGGATCCATTTCAGTTATAGGATGATTC; Mt42Xba, GGTCTAGACAGGACCATTTGGAAAAATC; Mt42Bam, GGGGATCCAGAATCATACACACATACAG; Mt401SA, GGACTAGTGGCGCGCCGGTTTGCCATGCTAT; Mt401BS, GGGGATCCATTTAAATCCATCAAGACTTGAATCT; Mt402BS, GGGATCCATTTAAATGGATGAATCTTTGTTGGCAA; Mt402SA, GGACTAGTGGCGCGCCTAAGTGCAATGGTTGG; 401N1, GAGAAGTGTGAGAGGGTATTAAAC; 402N2, CAGTTACCTACCTTACTCATCTG; 402N1, GGATGAATCTTTGTTGGCAA; 402N2, ACTTGCCGGTTTACCAACCT; MtACTIN2F, TGGCATCACTCAGTACCTTTCAACAG; MtACTIN2R, CCCAAAGCATCAAATAATAAGTCAACC.
Plasmids and vectors
For the construction of the RNAi vector sil401, a DNA fragment containing 330 bp of the 5′-untranslated region (UTR) including box1 sequences was amplified using primers Mt401SA and Mt401BS, while for the construction of vector sil402 a DNA fragment comprising the 5′UTR including box1 of MtENOD40-2 was amplified using primers Mt402BS and Mt402SA. Fragments were cloned into pGEM-T (Promega), yielding pGem401 and pGem402, respectively. Primers are provided with restriction enzyme recognition sites at their 5’ end to facilitate subsequent cloning.
The amplified fragments were released from pGEM-T and cloned in two orientations by two sequential cloning steps in pBS-d35S-RNAi (Limpens et al., 2004), using AscI and SwaI in the first cloning step and BamHI and SpeI in the second step, respectively. The inverted repeat preceded by the 35S promoter was released by digestion and subsequently cloned in the binary vector pRedroot (Limpens et al., 2004) using KpnI/PacI restriction enzymes delivering the pRRsil401 and pRRsil402 vectors, respectively. To be able to knock-down the expression of MtENOD40-1 and MtENOD40-2 simultaneously, the SpeI–NcoI/blunted fragment of pGem401 and the BamHI–EclI fragment of pGem402 were fused. The fragment obtained was then ligated into pGem402 from which the insert was removed after digestion with BamHI and SpeI, yielding pGem4012. The insert of pGem4012 was then cloned in inverted orientation and used to generate pRRsil401 and pRRsil402; these were then introduced into pBS-d35S-RNAi. The inverted repeat obtained was then cloned into pBS-d35S-RNAi as described above and subsequently in pRedroot, yielding pRRsil4012.
To obtain the MtENOD40-2 promoter sequence, a 1.8 kb DNA fragment upstream of the coding region of MtENOD40-2 was obtained by PCR using primers p402Hind and p402Bam with BAC (bacterial artificial chromosome) DNA as template. The sequences of the primers were designed based on available nucleotide sequences of the 3.9 kb DNA fragment from the BAC clone that hybridized to the M. truncatula EST BF519327. The obtained fragment was cloned into pCAMBIA1381Z digested with HindIII and BamHI.
Plant transformation
Vectors were transformed with Agrobacterium rhizogenes MSU 440, containing the helper plasmid pRiA4 (Sonti et al., 1995), by means of electroporation.
Agrobacterium rhizogenes-mediated root transformation was performed according to Limpens et al. (2004). Nitrogen-starved plants were inoculated with Sinorhizobium meliloti SM2011. To prevent nitrogen deficiency, 15 d after inoculation with bacteria, plants were provided with Fahreus medium supplemented with 1.5 mM ammonium nitrate.
RNA isolation and reverse transcription mediated-PCR
At least five M. truncatula roots 2 weeks after transformation with A. rhizogenes were selected by screening for red fluorescence and collected for RNA isolation using the RNeasy Plant Mini Kit (Qiagen). As control, a similar number of roots that did not show red fluorescence were collected. Synthesis of cDNA and subsequent semi-quantitative reverse transcriptase-PCR was performed as described (Ruttink et al., 2006). Successful removal of genomic DNA was checked prior to cDNA synthesis. The rest of the plants were inoculated with S. meliloti SM2011 (Limpens et al., 2004).
Histochemical analyses, in situ hybridization, and microscopy
Histochemical analyses of β-glucuronidase (GUS) activity was performed as described by Limpens et al. (2005). Sections of 21-d-old nodules were generated as decribed (Limpens et al., 2005). [35S]UTP-labelled RNA of MtENOD40-2 was produced by T7 RNA polymerase on MtENOD40-2 DNA cloned in plasmid pT7-5 (Scheres et al., 1990). Hybridization and subsequent detection and analyses of signal were performed as described (Limpens et al., 2005).
Sections of nodules for light and electron microscopy were obtained as described (Limpens et al., 2005).
Results
The Medicago truncatula genome contains two ENOD40 genes
The M. truncatula EST BF519327 has high homology to the 3′UTR of MtENOD40 (GenBank Accession no. X80262). To obtain genomic sequences corresponding to the BF519327-encoding gene, a BAC library of M. truncatula (Nam et al., 1999) was screened with BF519327. A 3.9 kb DNA fragment from a BAC clone was sequenced, and this contained a new ENOD40 gene, which has a region that is 100% identical to BF519327. Comparison of the nucleotide sequence of MtENOD40-2 and MtENOD40-1 (Fig. 1A) shows that the two genes only share 50% homology. Furthermore, the MtENOD40-2 gene contains the two conserved boxes (Fig. 1A, B) present in all leguminous ENOD40 genes identified to date, one of which contains an ORF for a small peptide (Fig. 1B). Whereas in all legumes the ENOD40 peptide contains the motif C-W-(X3)-I-H-G-S, in the MtENOD40-2 peptide the -I-H-G-S amino acid sequence is substituted by -I-Y-D (Fig. 1C).
Comparison of MtENOD40 sequences. (A) Nucleotide sequence alignment of MtENOD40-1 (lower case) and MtENOD40-2 (upper case). Box1 and box2 sequences are boxed. Within box2, the nucleotide sequences conserved in all ENOD40 sequences (Kouchi et al., 1999) are in bold. Nucleotide sequences of primers for gene-specific knock-down are underlined. Primer names are indicated on the right. (B) Nucleotide sequence alignment of box1 and amino acid sequence alignment of the ORF in box1 of legume ENOD40 genes. Plant species abbreviations and GenBank accession nos: MsENOD40, Medicago sativa (L32806); MtENOD40, Medicago truncatula (1, X80264; 2, X80262); VsENOD40, Vicia sativa (X83683); PsENOD40, Pisum sativum (X81064); TrENOD40, Trifolium repens (1, AF426838; 2, AF426839; 3, AF426840); SrENOD40, Sesbania rostrata (Y12714); PvENOD40, Phaseolus vulgaris ((X86441); LjENOD40, Lotus japonicus (1, AF013594; 2, AJ271788); GmENOD40, Glycine max (1, X69154; 2, D13503).
On a Southern blot containing EcoRI-digested M. truncatula genomic DNA, the MtENOD40-2 probe hybridized to two fragments, which is expected as MtENOD40-2 contains one EcoRI site. In contrast, the MtENOD40-1 probe hybridized to one fragment, as MtENOD40-1 does not contain an EcoRI site (data not shown). Thus these data show that the M. truncatula genome contains two ENOD40 genes.
MtENOD40-2 is induced during nodulation
To determine whether MtENOD40-2 is expressed in nodule primordia and nodules, like MtENOD40-1, hairy roots containing a 1.8 kb DNA fragment upstream of the coding region of MtENOD40-2 to drive the GUS reporter gene were analysed for GUS activity 2 d and 21 d after inoculation with S. meliloti. In sections of roots collected 2 d post-inoculation, GUS activity was present in dividing cortical cells, indicating that MtENOD40-2 is expressed in cells of the nodule primordium (Fig. 2C). Whole-mount staining for GUS activity of nodules showed that GUS activity is detected near the apex of the nodule and in vascular bundles (Fig. 2D). To localize the site of expression of MtENOD40-2 precisely in the nodule, in situ hybridization using [35S]UTP-labelled antisense MtENOD40-2 RNA was conducted (Fig. 2A, B). This showed that MtENOD40-2 is expressed in cells of the infection zone (Fig. 2B, IZ). Thus, the MtENOD40-2 expression pattern is similar to the MtENOD40-1 expression pattern (Crespi et al., 1994). Furthermore, the GUS expression studies are consistent with the in situ hybridization data, indicating that the 1.8 kb DNA fragment used contains the elements required for the regulation of MtENOD40-2 expression. Based on the combination of expression data and the sequence homology between both genes, it is likely that MtENOD40-2 is functional in nodule initiation and development.
Expression of MtENOD40-2 during nodulation. (A, B) In situ hybridization of a longitudinal section hybridized to [35S]UTP-labelled antisense MtENOD40-2 RNA. Bright field micrograph (A). Dark field micrograph (B), where the signal appears as white grains. Signal is present in the infection zone (IZ) and absent from the meristem (M). Bar=200 μm. (C) Histochemical localization of GUS activity in a semi-thin (7 μM) section of pMtENOD40-2:GUS roots, 2 d after inoculation with S. meliloti. Dividing cortical cells are indicated by an asterisk (*). Bar=25 μm. (D) Whole-mount detection of pMtENOD40-2:GUS activity in 21-d-old nodules, showing promoter activity in the apical part of the nodule and in vascular bundles. Bar=0.5 mm.
Gene specific knock-down of MtENOD40-1 and MtENOD40-2
To find out whether MtENOD40-1 and MtENOD40-2 are both required for nodule formation, the effect of reduction in expression of each gene individually on nodule formation has been investigated.
To reduce MtENOD40-1 and MtENOD40-2 gene expression, A. rhizogenes-mediated RNAi was applied in M. truncatula hairy roots (Limpens et al., 2004). To this end, one vector (pRRsil401) was designed that is expected to lead to a reduction in expression of MtENOD40-1, and a second vector (pRRsil402) that is expected to lead to a reduction in MtENOD40-2 expression. To knock-down transcription of both genes simultaneously, a third vector (pRRsil4012) was used (Materials and methods).
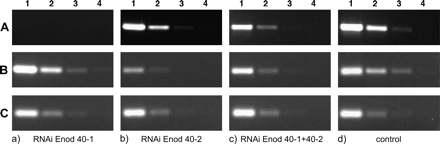
Two-week-old transgenic roots were analysed for the levels of MtENOD40-1 and MtENOD40-2 transcripts by RT-PCR using MtENOD40-1- and MtENOD40-2-specific primers (Fig. 3). The transcript level of MtENOD40-1 was reduced about 25-fold in RNA isolated from MtENOD40-1 RNAi roots as compared with that of control roots, that did not show red fluorescence (Fig. 3A, compare columns a and d), while MtENOD40-2 expression was not altered in MtENOD40-1 RNAi roots (Fig. 3B, compare columns a and d). In RNA isolated from MtENOD40-2-silenced roots, the transcript level of MtENOD40-2 is reduced 5- to 25-fold compared with the transcript level in control roots (Fig. 3B, compare columns b and d), while the level of MtENOD40-1 in MtENOD40-2 RNAi and control roots is similar (Fig. 3B, compare columns a and d). Thus by using the pRRsil401 and pRRsil402, the expression of the two related genes MtENOD40-1 and MtENOD40-2 can be knocked down specifically. In roots containing RRsil4012 DNA, the level of the transcript of MtENOD40-1 and MtENOD40-2 is reduced >5-fold compared with the transcript levels of MtENOD40-1 and MtENOD40-2 in control roots (Fig. 3A, compare columns c and d; Fig. 3B, compare columns c and d). This shows that application of pRRsil4012 leads to a reduction in expression of both MtENOD40-1 and MtENOD40-2.
RT-PCR analyses of MtENOD40-1 and MtENOD40-2 expression in knock-down roots using gene-specific primers. (A) MtENOD40-1 RNA level in MtENOD40-1 RNAi (column a), MtENOD40-2 RNAi (column b), and double RNAi (column c). Reduction of MtENOD40-1 RNA level in MtENOD40-1 RNAi and double RNAi, but not in MtENOD40-2 RNAi (column b) roots, compared with control roots (column d). (B) MtENOD40-2 RNA level in MtENOD40-1 RNAi (column a), MtENOD40-2 RNAi (column b), and the double RNAi (column c). The MtENOD40-2 RNA level is reduced in MtENOD40-2 RNAi and double RNAi, but not in MtENOD40-1 RNAi roots, compared with control roots (column d). (C) Mtactin2 RNA levels. Amplification is shown in 0- (1), 5- (2), 25- (3), and 125-fold dilutions (4) of the cDNA mix at a fixed number of cycles; 30 cycles for MtENOD40-1, 30 cycles for MtENOD40-2, and 22 cycles for Mtactin.
Reduced nodule number in MtENOD40-1 and MtENOD40-2 knock-downs
Nodules formed on roots in which the MtENOD40-1 and MtENOD40-2 genes are knocked down were determined 3 weeks after inoculation (Table 1).
Effect of RNAi on number of nodules
| Nodules/root | Reduction | |
| MtENOD40-1 RNAi | 3.2±0.3 (n=51) | 46.4% (P <0.001) |
| Wild type | 5.9±0.2 (n=61) | |
| MtENOD40-2 RNAi | 3.4±0.2 (n=55) | 38.5% (P <0.01) |
| Wild type | 5.5±0.2 (n=62) | |
| MtENOD40-1 and -2 RNAi | 1.5±0.2 (n=60) | 75.0% (P <0.000001) |
| Wild type | 6.0±0.3 (n=62) |
| Nodules/root | Reduction | |
| MtENOD40-1 RNAi | 3.2±0.3 (n=51) | 46.4% (P <0.001) |
| Wild type | 5.9±0.2 (n=61) | |
| MtENOD40-2 RNAi | 3.4±0.2 (n=55) | 38.5% (P <0.01) |
| Wild type | 5.5±0.2 (n=62) | |
| MtENOD40-1 and -2 RNAi | 1.5±0.2 (n=60) | 75.0% (P <0.000001) |
| Wild type | 6.0±0.3 (n=62) |
Effect of RNAi on number of nodules
| Nodules/root | Reduction | |
| MtENOD40-1 RNAi | 3.2±0.3 (n=51) | 46.4% (P <0.001) |
| Wild type | 5.9±0.2 (n=61) | |
| MtENOD40-2 RNAi | 3.4±0.2 (n=55) | 38.5% (P <0.01) |
| Wild type | 5.5±0.2 (n=62) | |
| MtENOD40-1 and -2 RNAi | 1.5±0.2 (n=60) | 75.0% (P <0.000001) |
| Wild type | 6.0±0.3 (n=62) |
| Nodules/root | Reduction | |
| MtENOD40-1 RNAi | 3.2±0.3 (n=51) | 46.4% (P <0.001) |
| Wild type | 5.9±0.2 (n=61) | |
| MtENOD40-2 RNAi | 3.4±0.2 (n=55) | 38.5% (P <0.01) |
| Wild type | 5.5±0.2 (n=62) | |
| MtENOD40-1 and -2 RNAi | 1.5±0.2 (n=60) | 75.0% (P <0.000001) |
| Wild type | 6.0±0.3 (n=62) |
Whereas an average of 3.2 nodules/MtENOD40-1 RNAi root was formed, 5.9 nodules/root are formed on control roots (46.4% reduction). On MtENOD40-2 RNAi roots, the average number of nodules per root was 3.4, which corresponds to a reduction in nodule numbers of 38.5%. These data indicate that MtENOD40-1 as well as MtENOD40-2 is involved in nodule initiation. On roots, in which the expression of both MtENOD40-1 and MtENOD40-2 is reduced, the average number of nodules per root is 1.5 (75% reduction). Thus knocking down of the expression of both genes has an additive effect, suggesting that MtENOD40 acts in a dose-dependent manner.
Reduced MtENOD40-1/40-2 expression affects symbiosome development
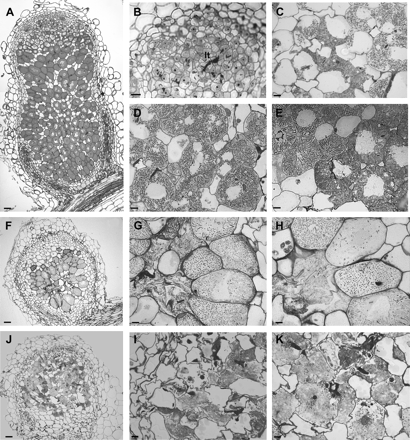
To determine whether MtENOD40-1 and MtENOD40-2 are required for nodule development, nodules formed on MtENOD40-1 and MtENOD40-2 RNAi plants were analysed in more detail. Whereas 3-week-old nodules on control plants are rod shaped (Fig. 4A), the nodules formed on MtENOD40-1 or MtENOD40-2 RNAi plants are spherical and small (Fig. 4E). Longitudinal sections were prepared from control nodules and nodules of MtENOD40-1 or MtENOD40-2 RNAi roots to analyse their cytology.
Histology of 3-week-old RNAi nodules (F–K) compared with control nodules (A–E). Light microscopy of a control nodule (A–E). (A) Median longitudinal section of a 3-week-old nodule. (B) Magnification of the meristem and the distal part of the infection zone of a wild-type nodule, where bacteria are released from infection threads (It). (C) Magnification of the infection zone, where short rod-shaped bacteriods are present at the distal part, and (D) long rod-shaped bacteroids in the proximal part. (E) Magnification of the fixation zone containing cells fully packed with elongated bacteroids. (F) Light microscopic analyses of a longitudinal section of an aberrant MtENOD40-1 RNAi nodule. Note that the zonation is not clear. (G) A senescent nodule wherein cells in the middle of zone III lost turgor and collapsed. (H) Note the size of bacteroids compared with bacteroids in (E). (J) A dead nodule recolonized by saprophytic rhizobia. (I) Magnification of collapsed cells being repopulated with bacteria. (K) Release of rhizobia from intracellular colonies. Bars=20 μm.
Analyses of sections of MtENOD40-1 RNAi nodules showed that in about half of the MtENOD40-1 RNAi nodules (Fig. 4F, J), the zonation of the central tissue cannot be recognized (compare Fig. 4A–D and 4F, J). The majority of these nodules were senescent. Some cells in the proximal part are repopulated by rhizobia. These are rod shaped, and electron microscopy (EM) studies show that they lack a plant-derived membrane and the ultrastructural differentiation features of bacteroids. Therefore, the bacteria colonize cells in a saprophytic manner (Timmers et al., 2000; Fig. 4J, K). In about half of the MtENOD40-2 RNAi and 40% of the nodules from plants in which the expression of both genes was reduced, growth disturbances are similar to those observed in the MtENOD40-1 RNAi nodules.
Among the nodules studied, nodules that were less disturbed in their development were also identified (Fig. 4F–H). In cells of the infection zone of these nodules, a few bacteria are released from the infection threads and can be recognized as small rod-shaped bacteroids. However, bacteroids remain short and rod shaped. At later stages of development, in the middle of the nodule lysed cells with an irregular shape that lost turgor are present (Fig. 4F, G) as well as senescent cells (Fig. 4G, H). Thus light microscopy (LM) analyses showed that bacteroid development was impaired. To identify which step in bacteroid development is affected, bacteroids in these nodules were studied by EM.
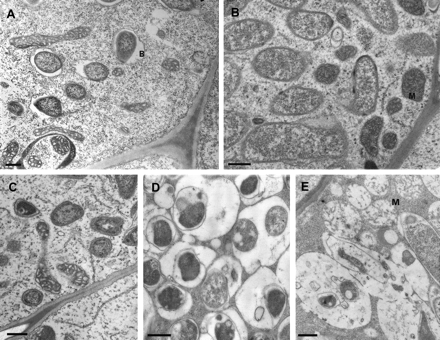
In MtENOD40-1 RNAi nodules (Fig. 5A, B), bacteria are released from infection threads, and EM analyses revealed that each bacteroid was surrounded by symbiosome membrane as in the wild type (Fig. 5C). In contrast to wild-type nodules, bacteroid development was arrested at stage II–III and never developed into bacteroids of stage IV (Fig. 5). Further, in cells of the fixation zone, bacteroids undergo premature senescence (Fig. 5D). This is characterized by the presence of electron-dense cytoplasm, an enlarged peribacteroid space, and an irregular shape. Furthermore, in cells of this zone, fusion of symbiosomes leads to the formation of vacuole-like structures with lysis of bacteroids that are trapped inside (Fig. 5E). The latter is a typical feature for (premature) symbiosis termination (Vance and Johnson, 1983; Vasse et al., 1990). The bacteroid lysis was followed by destruction of mitochondria, as cells contained swollen organelles with degraded matrix and a very low number of cristae (Fig. 5E). These observations show that senescence of both partners occurs concomitantly. Analyses of MtENOD40-2 RNAi and nodules in which the expression of both MtENOD40 genes is reduced also showed that bacteroid development was blocked at stage II–III. These data indicate that reduction of the MtENOD40-1 and MtENOD40-2 expression levels in nodules interfered with bacteroid development. Strikingly, irrespective of which gene is knocked down, the percentage of the nodules with growth disturbances is similar (∼50%). This indicates that MtENOD40-1 and MtENOD40-2 genes are not acting redundantly.
Ultrastructure of control (A and B) and 3-week-old RNAi nodules (C, D, and E). (A) Wild-type nodules; young bacteroids in the distal part of the infection zone. (B) Wild-type nodules; proximal part of the infection zone. (C) RNAi nodules; infection zone. (D) Bacteroids with a large peribacteroid space, fusing. (E) Lysis of bacteroids entrapped inside a vacuole-like structure. Note swollen mitochondria of the host cell (B, bacteroids; M, mitochondria. Bars= 500 nm).
Discussion
Here the identification of a second MtENOD40 gene, MtENOD40-2, and the involvement of this gene in nodule formation were described.
MtENOD40-2 contains the two regions, box1 and box2, that are conserved among all ENOD40 genes known so far. However, whereas all legume ENOD40 genes contain an ORF encoding a peptide with the conserved amino acid motif -C-W-(X3)-I-H-G-S, the peptide encoded within the ORF of MtENOD40-2 lacks the three C-terminal amino acids -H-G-S. It is not clear whether the activity of ENOD40 is determined by the peptide encoded within box1 of ENOD40. Hence, the significance of the change in amino acid order of MtENOD40-2 peptide with respect to activity of MtENOD40-2 remains unknown.
ENOD40 gene expression has been shown to be highly induced during the interaction of the roots of legumes with Rhizobium. Here, it is shown that MtENOD40-2 is expressed in the nodule primordium and in the infection zone of the nodule, like MtENOD40-1. Co-localization of different ENOD40 genes within one species has also been shown in Medicago sativa (Fang and Hirsch, 1998), L. japonicus (Flemetakis et al., 2000), and T. repens (Varkonyi-Gasic and White, 2002). Although the levels of MtENOD40-1 and MtENOD40-2 expression in nodules have not been compared, the detection of 42 ESTs for MtENOD40-1 among several cDNA libraries of M. truncatula nodules (http://www.tigr.org/tigr-scripts/tgi/T_index.cgi?species=medicago; http://medicago.toulouse.inra.fr/Mt/EST/), compared with one for MtENOD40-2, strongly indicates that MtENOD40-1 is much more highly expressed in nodules than MtENOD40-2.
The spatial and temporal expression pattern of MtENOD40-1 and MtENOD40-2 during nodule formation suggests a role for these genes in this process. In M. truncatula, knock-down of MtENOD40-1 expression led to a 50% reduction in the number of nodules (Charon et al., 1999), and RNAi of LjENOD40-1 in L. japonicus led to an even more drastic reduction in nodule numbers. Thus, these data all show that ENOD40 is involved in nodule initiation. However, since in both experiments the introduced ENOD40-1 DNA contained the conserved box2 sequences, the observed reduction in nodule number cannot be assigned to the reduction of ENOD40-1, per se. Here it is shown, by using a gene-specific knock-down, that MtENOD40-1 and MtENOD40-2 are both involved in nodule initiation. The observation that a decrease in expression levels of either MtENOD40-1 or MtENOD40-2 reduces nodule initiation suggests that the two MtENOD40 genes do not act redundantly in nodule initiation. Further, as a reduction of the expression of both MtENOD40 genes leads to a higher reduction of nodule initiation, the effect on nodule initiation of the MtENOD40 genes is synergistic. Therefore, it is proposed that the effect of MtENOD40 on nodule initiation is dose dependent. This dose-dependent effect of ENOD40 genes on nodule initiation explains why no mutant in ENOD40 came out of the genetic screens for mutants impaired in nodule initiation. In both L. japonicus and M. truncatula, a reduction, but not a complete knock-down, in ENOD40 expression leads to significant inhibition of nodule formation. Therefore, the present results and the results in L. japonicus (Kumagai et al., 2006) strongly suggest that ENOD40 genes are essential for nodule initiation.
Furthermore, it is shown that in all knock-downs tested, the percentage (50%) of aberrant nodules formed is similar, and that all aberrant nodules show an impaired bacteroid developmental progression from stage II to III. This suggests that the MtENOD40 genes do not act redundantly in bacteroid development and, in contrast to their involvement in nodule initiation, neither acts synergistically. This shows that both MtENOD40 genes are essential for bacteroid development.
It is likely that in the aberrant nodules, bacteroids are unable to fix nitrogen. In the nodules that do not show an impaired bacteroid development, it is expected that bacteroids are able to fix nitrogen. The present data are consistent with the observations in nodules formed on RNAi LjENOD40-1 plants (Kumagai et al., 2006). Some of these L. japonicus nodules are also white and small, suggesting an impaired nodule development.
Here it is shown that MtENOD40-1 and MtENOD40-2 are both required for bacteroid development. This observation therefore offers an opportunity for unravelling the nature of the biological activity of ENOD40.
This work was supported by the Netherlands Organization for Scientific Research (WOTRO 86-160, XW) and the Erasmus Exchange Program (AI and CG).




![Expression of MtENOD40-2 during nodulation. (A, B) In situ hybridization of a longitudinal section hybridized to [35S]UTP-labelled antisense MtENOD40-2 RNA. Bright field micrograph (A). Dark field micrograph (B), where the signal appears as white grains. Signal is present in the infection zone (IZ) and absent from the meristem (M). Bar=200 μm. (C) Histochemical localization of GUS activity in a semi-thin (7 μM) section of pMtENOD40-2:GUS roots, 2 d after inoculation with S. meliloti. Dividing cortical cells are indicated by an asterisk (*). Bar=25 μm. (D) Whole-mount detection of pMtENOD40-2:GUS activity in 21-d-old nodules, showing promoter activity in the apical part of the nodule and in vascular bundles. Bar=0.5 mm.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/58/8/10.1093/jxb/erm072/2/m_jexboterm072f02_4c.gif?Expires=1716314977&Signature=ekcHlVE3qbnNbM~8J0u4-6CCpYGNMlsUKIKarLZ6tJzAH3oZuTqFF2~GjjRBAf8V7PPvEomtBuB5S6qA~2NDb-Ag-wsIALqogECe-6YZA6R9YID1bbEQWRphL2R8EoYAyUVCZqlJcKSYMc0NbJkL7a4bSNK73kbFSW40M2cZRvlEG5zguSEQopIjmvt9~Iake3Itw~fQiwskJL~W4D0jntoISo2NMrHfgtV3ghTxvrJij-ry0MJtuWQJzbR1tbUILEiVXqsLJffFSyZUk2j3ko2uORFAQzcu4Wuv1-8m-0fHCJ5fjhK4sUPngJAQZbHVnKQw4S8vtRD4z-r9r0ir2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments