-
PDF
- Split View
-
Views
-
Cite
Cite
Matthias A. Karajannis, Geneviève Legault, Mari Hagiwara, Filippo G. Giancotti, Alexander Filatov, Anna Derman, Tsivia Hochman, Judith D. Goldberg, Emilio Vega, Jeffrey H. Wisoff, John G. Golfinos, Amanda Merkelson, J. Thomas Roland, Jeffrey C. Allen, Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas, Neuro-Oncology, Volume 16, Issue 2, February 2014, Pages 292–297, https://doi.org/10.1093/neuonc/not150
Close - Share Icon Share
Abstract
Activation of the mammalian target of rapamycin (mTOR) signaling pathway is thought to be a key driver of tumor growth in Merlin (NF2)-deficient tumors. Everolimus is an oral inhibitor of mTOR complex 1 (mTORC1) with antitumor activity in a variety of cancers.
We conducted a single-institution, prospective, 2-stage, open-label phase II study to estimate the response rate to everolimus in neurofibromatosis type 2 (NF2) patients with progressive vestibular schwannoma (VS). Ten eligible patients were enrolled, including 2 pediatric patients. Everolimus was administered at a daily dose of 10 mg (adults) or 5 mg/m2/day (children <18 y) orally in continuous 28-day courses, for up to 12 courses. Response was assessed every 3 months with MRI, using 3-dimensional volumetric tumor analysis, and audiograms. Nine patients were evaluable for the primary response, defined as ≥15% decrease in VS volume. Hearing response was evaluable as a secondary endpoint in 8 patients.
None of the 9 patients with evaluable disease experienced a clinical or MRI response. No objective imaging or hearing responses were observed in stage 1 of the trial, and the study was closed according to predefined stopping rules.
Everolimus is ineffective for the treatment of progressive VS in NF2 patients. We are currently conducting a pharmacokinetic/pharmacodynamic (“phase 0”) study of everolimus in presurgical VS patients to elucidate the biological basis for apparent treatment resistance to mTORC1 inhibition in these tumors.
Neurofibromatosis type 2 (NF2) is an autosomal dominant genetic disorder caused by inactivation of the NF2 gene located on chromosome 22q and acts as a tumor suppressor.1 NF2 patients develop multiple central and peripheral nervous system tumors, including most commonly VS and other cranial nerve schwannomas, as well as meningiomas and ependymomas.2 This progressive tumor burden typically presents early in life and leads to substantial morbidity, including progressive sensorineural hearing loss, as well as other neurological impairments and significantly reduced life expectancy. As a consequence, NF2 patients suffer from communication impairment and are at increased risk for negative psychosocial repercussions, including depression.3,4
Traditional therapeutic options, consisting of surgery and/or radiation therapy, are generally unsuccessful in reversing existing neurological damage and frequently cause additional morbidity. Conventional chemotherapies have not been used to treat NF2 patients due to concerns about unacceptable toxicities, such as neuro- and/or ototoxicity, as well as their mutagenic properties, which are of special concern in NF2 patients. During recent years, our understanding of NF2 tumor biology has increased tremendously, leading to intensified efforts to identify novel molecular targets in preclinical studies, followed by validation in clinical trials.
The mammalian target of rapamycin (mTOR) signaling pathway has been identified as a major mediator of tumor suppressor activity of moesin-ezrin-radixin-like protein (Merlin) and provides an attractive therapeutic target in NF2.5 Recent studies have revealed that loss of Merlin activates mammalian target of rapamycin complex 1 (mTORC1) signaling. Mammalian target of rapamycin regulates essential signal-transduction pathways, linking growth stimuli to cell-cycle progression, and integrates signals involving nutrient availability, energy status, and stress.6,7 Inhibition of mTORC1 by rapamycin reduces the growth of Merlin-deficient arachnoidal, meningioma, and schwannoma cells.8,9 In addition, targeting mTORC1 may inhibit production of vascular endothelial growth factor (VEGF) and therefore reduce tumor angiogenesis.10 This mechanism of action is relevant to vestibular schwannoma (VS) because therapeutic inhibition of VEGF using bevacizumab is a clinically validated treatment for VS that can lead to dramatic responses.11 Everolimus (RAD001), a rapamycin analog, is an orally administered mTOR kinase inhibitor. Everolimus is approved by the FDA for patients with advanced renal cell carcinoma (after failure of treatment with sunitinib or sorafenib) and advanced pancreatic neuroendocrine tumors, as well as subependymal giant cell tumors and angiomyolipoma associated with tuberous sclerosis complex.12–15 Similar to rapamycin, everolimus inhibits mTORC1 via binding to cyclophilin FK506 binding protein 12 (FKBP12) but has improved oral bioavailability and pharmacokinetics, as well as reduced immunosuppressive activity.16 Everolimus reduces cell proliferation, cell growth, angiogenesis, and glucose uptake, inhibits expression of hypoxia-inducible factor 1, and reduces VEGF expression in animal models.17,18 Everolimus may also reduce the amount of tumor vasculature and VEGF production without adversely affecting vascular permeability.10
Everolimus is generally well tolerated, with the most frequently reported adverse events including rash, mucositis, fatigue, and headache. Noninfectious pneumonitis has been reported with everolimus but is rarely severe and generally reversible. The pharmacokinetics and safety of everolimus in children have been previously reported in a pediatric phase I study.19
Based on this encouraging preclinical data and a favorable safety profile, we conducted a single-institution, prospective, 2-stage, open-label phase II study to estimate the response rate to everolimus in NF2 patients with progressive VS and other NF2-related tumors.
Methods
Patient Eligibility and Enrollment
Adult and pediatric patients of ≥3 years of age and with a body surface area (BSA) of ≥0.5 m2 were eligible. Inclusion criteria also mandated patients to meet the revised National Institutes of Health diagnostic criteria for NF220 and to have at least one NF2-related VS with either volumetric progression or significant hearing decline over the preceding 12 months designated as the primary target tumor. For eligibility, progressive tumor growth was defined as increase in tumor size of at least 2 mm in greatest diameter on conventional MRI20 or a >10% volume increase by 3D volumetrics. Progressive hearing loss for eligibility was defined as a drop in pure tone average (PTA) of ≥10 dB at ≥2 nonconsecutive or consecutive frequencies or a drop in word recognition score (WRS) below the 95% critical difference threshold.21 Histological confirmation was not required, as tumor biopsies are rarely indicated in this disease. Additional key eligibility criteria included Karnofsky/Lansky performance status ≥50%; adequate bone marrow, renal, and hepatic function; and stable neurological deficits for ≥1 week, if applicable. Key exclusion criteria included major surgery or significant traumatic injury within 4 weeks prior to start of study drug, prior treatment with an mTOR inhibitor (eg, sirolimus, temsirolimus, everolimus), impairment of gastrointestinal function or gastrointestinal disease that may significantly alter the absorption of everolimus, active bleeding diathesis, and pregnancy or breast-feeding in female patients.
A standard 12-lead electrocardiogram was performed during screening. Everolimus has been associated with disease reactivation in hepatitis B/C carriers, and assessment of hepatitis B/C medical history and risk factors was therefore performed for all patients at screening. Hepatitis B/C testing was performed in all at-risk patients, and if carrier status was confirmed, patients were started on appropriate antivirals beginning 1–2 weeks prior to the first dose of everolimus, when applicable.
This study was conducted under a protocol approved by the institutional review board of NYU Langone Medical Center and registered at ClinicalTrials.gov (NCT01419639). Informed consent was obtained from the patients and guardians in accordance with institutional policies. All consecutive patients who met study entry criteria and who consented to participate were enrolled.
Study Design
This was a single-institution, prospective, 2-stage, phase II open label study. The primary objective was to estimate the objective response rates to everolimus in patients with NF2-related VS. Secondary objectives included toxicity assessment of everolimus given daily in patients with NF2 and estimation of the association of objective measures of response on MRI (ie, volumetric tumor analysis) with clinical measures of response (ie, audiogram) in patients with VS. A 2-stage Simon design22 was used to test the null hypothesis of a response rate ≤5%, against the alternative hypothesis of a response rate ≥25%. Nine patients were to be enrolled in stage 1. If at least 1 patient of these 9 had a volumetric response in stage 1, at any given evaluation point, an additional 8 patients were to be enrolled in stage 2. The overall alpha level for this design was 0.05 with a power of 80%. Everolimus was to be considered effective and of interest for further study if, after successful completion of both stages, the cumulative number of responses was ≥3.
Treatment
Everolimus was provided by Novartis and administered in continuous 4-week courses. Pediatric patients <18 years of age received 5 mg/m2/day according to their BSA: 2.5, 5, 7.5, and 10 mg p.o. once daily for BSAs of 0.50–0.99, 1.00–1.49, 1.5–1.99, and ≥2 m2, respectively. Adults ≥18 years of age received a standard recommended adult dose of 10 mg p.o. once daily. For drug-induced stomatitis/oral mucositis/mouth ulcers due to everolimus, local supportive care was recommended, modified according to severity: for mild toxicity (grade 1), conservative measures such as nonalcoholic mouthwash or salt-water (0.9%) mouthwash several times a day until resolution; for more severe toxicity (grade ≥2), topical analgesic mouth treatments (ie, local anesthetics such as benzocaine, butyl aminobenzoate, tetracaine hydrochloride, menthol, or phenol) with or without topical corticosteroids, such as triamcinolone oral paste 0.1%.
Routine clinical evaluations, including a complete physical examination, complete blood count with differential, comprehensive metabolic panel, uric acid, urinalysis, fasting serum lipid profile (triglycerides, total cholesterol, high-density lipoprotein, and low-density lipoprotein), and serum pregnancy test (for females of child-bearing potential) were performed at baseline, after day 1, at weeks 1, 2, and 4 of the first course, and every 4 weeks thereafter. Patients were allowed to remain on study until disease progression or unacceptable toxicity occurred. Adverse events were graded using version 3.0 of the National Cancer Institute Common Toxicity Criteria (CTCAE). For patients who were unable to tolerate the protocol-specified dosing schedule, drug dosing was interrupted or modified according to protocol-prespecified rules, with a maximum of 2 dose reductions allowed per patient. For dose delays of >21 days from the intended day of the next scheduled dose, the patient was permanently discontinued from the study.
Response Evaluation
Brain and spine MRIs (in patients with spinal tumors) with dynamic contrast-enhanced perfusion and 3D volumetrics and audiograms (in patients with VS, although not required for patients with no measurable hearing) were performed at baseline and after every third course, that is, every 12 weeks. Three-dimensional tumor volumetrics were obtained on postcontrast, T1-weighted magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequences with a 1-mm slice thickness and no gap, using semiautomated segmentation software (Vitrea platform). Serial audiological evaluations were used to assess hearing response, including determination of pure-tone thresholds and WRS. WRS was tested using the 50-item recorded Central Institute for the Deaf CID-W22 monosyllable word list. WRS represents the most functionally relevant measure of hearing in NF2 patients and is therefore recommended as a trial endpoint.21,23 PTA was calculated by the mean of the individual threshold frequencies at 500, 1000, 2000, and 4000 Hz and was recorded for each ear. An increase of ≥10 dB in the PTA between any follow-up assessment and the baseline value was considered hearing deterioration, while a decrease by ≥10 dB indicated a clinically significant improvement, as previously suggested.21
The primary efficacy endpoint was best radiographic tumor response (maximum tumor shrinkage) during the first 12 courses of treatment; the secondary efficacy endpoint was hearing response. It has been previously shown that 3D volumetrics for tracking VS are more sensitive than linear measurements, with the latter tending to underestimate growth rates.24 Volumetrics have therefore become the modality of choice for defining and assessing radiographic response in NF2 clinical trials.21,23 For study purposes, a ≥15% reduction in tumor volume in any of the target tumors constituted a partial response. Complete disappearance of any of the target tumors constituted a complete response. Stable disease was defined as <15% growth or shrinkage in tumor volume. Hearing response was defined as an improvement in the WRS above the 95% critical difference threshold, compared with the baseline audiogram at the initiation of treatment,21,25,26 and as recommended for the NF2 hearing endpoint.21 On the contrary, volumetric growth of the primary target tumor by ≥15% defined volumetric progression and instigated discontinuation of therapy. Similarly, in comparison with the baseline audiogram, a decrease in WRS below the 95% critical difference threshold constituted hearing progression. All additional target tumors were monitored for growth or response, but any progression in those tumors did not trigger treatment termination.
Statistical Analysis
Disease and patient characteristics at baseline were summarized using descriptive statistics. Overall response rates were estimated with exact Clopper–Pearson 95% confidence limits.
Progression-free survival (PFS) was measured from first date of study drug to date of volumetric or hearing progression, whichever event occurred first. PFS was summarized using the Kaplan–Meier methods for overall PFS. Point estimate for PFS with 95% confidence intervals was estimated.
Results
Patients
Ten eligible patients were enrolled between January and June 2012. There were 7 males (70.0%) and 3 females (30.0%), and participants were a median age of 27 years at enrollment (range, 12–44), with 2 who were pediatric patients <18 years of age. All but 1 patient (patient 5; familial NF2) had sporadic NF2. All patients or their legal representative provided written informed consent for treatment. One patient decided to terminate study participation after 3 weeks for personal reasons and was therefore not evaluable for response, leaving 9 patients evaluable for the primary volumetric response and 8 remaining hearing patients evaluable for hearing response. Stage 2 of the study was never opened for enrollment, as no objective response was observed in stage 1, terminating accrual after a total of 9 evaluable patients was reached. The majority (78%) of evaluable patients had received prior treatment, most commonly lapatinib and/or bevacizumab, for NF2-related tumors. Characteristics of all evaluable patients are recorded in Supplementary Data.
Treatment
The median number of courses received by the 9 evaluable patients was 6 four-week courses (range, 3–12). Three patients (patients 1, 2, and 3) came off trial due to tumor progression after 9, 3, and 5 courses, respectively. Three other patients (patients 7, 8, and 9) discontinued treatment after 6, 6, and 3 courses, respectively, because of patient preference due to lack of volumetric and/or hearing response. Two patients completed 12 courses, at which time one (patient 4) met criteria for hearing progression and the other (patient 6) had stable disease (both volumetrically and hearing-wise). One patient came off study after 3 courses due to toxicity, that is, pneumonia and decreased pulmonary function (patient 5).
Toxicity
All 10 enrolled subjects were available for toxicity monitoring. Two patients (20.0%) experienced grade 3 toxicities: transient azoospermia (patient 1, resolved after drug discontinuation) and fatigue (patient 7), at least possibly related to study drug. One patient with prior history of aspiration pneumonias and preexisting restrictive lung disease came off study after 3 courses due to toxicity: pneumonia and decreased pulmonary function. Observed toxicity was otherwise minor (CTCAE 3.0 grades 1 and 2) and most commonly included rash (80%) and mouth ulcers (100%), and less commonly included fatigue (50%), headache (60%), anemia (50%), and cholesterol elevation (70%). No grade 4 toxicity was observed.
Volumetric and Hearing Responses
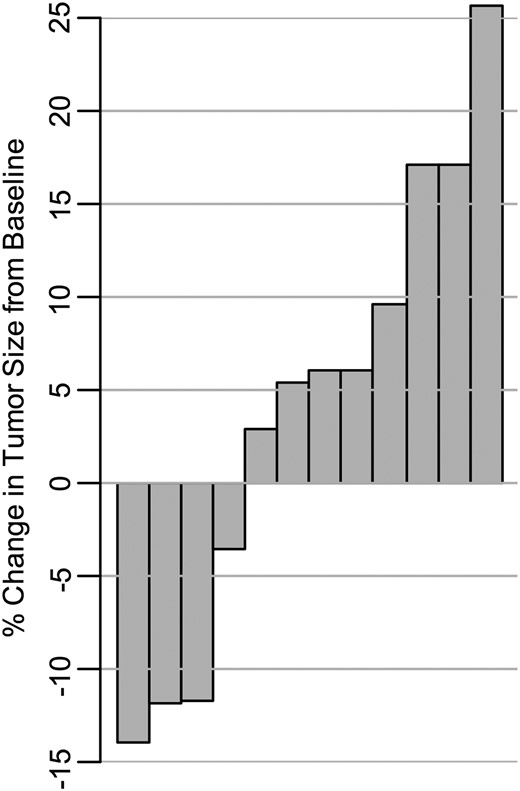
Baseline patient characteristics and responses to treatment are summarized in Supplementary Data, respectively, with additional details included in Supplementary Data. All 9 evaluable patients had VS as the primary target tumor, with 2 patients (patients 5 and 9) having bilateral VS as the target tumor, and another patient (patient 1) with a right C1 nerve root tumor in addition to his primary right VS, totaling 12 target tumors. Patient 1 experienced the best volumetric change, of −14.0% (shrinkage) in his right C1 tumor, as well as the largest target tumor growth, of +25.7% (progression) in his VS tumor compared with the baseline measurement (see Fig. 1). Although 4 target tumors, including 3 primary target tumors, demonstrated some level of tumor reduction during therapy, they did not reach criteria for volumetric response in any evaluable subjects: median of shrinkage was −11.9%, with a range from −3.6% to −14.0%. The 3 patients (patients 1, 2, and 3) who discontinued study drug due to tumor progression suffered from volumetric growths of +25.7%, +17.1%, and +17.1%, respectively.
Waterfall plot of the percentage of change in tumor volume, from baseline, for each evaluable tumor (n = 12). Each column represents a volumetrically measurable individual VS with the exception of 1 right C1 nerve root tumor in patient 1. For each tumor, the best response while on study is shown. For tumors that did not show any volume reduction, the largest percentage of volumetric growth during therapy is indicated.
The distributions of the PTA and WRS for each ear at enrollment are shown in Supplementary Data. None of the patients experienced an improvement in their WRS sufficient to meet the definition of a clinical response, as established a priori. One patient (patient 4) completed 12 courses, at which time he met criteria for hearing progression. Two other patients (patients 2 and 4) sustained deterioration in their mean PTA after 3 and 9 months, of +12.5 and +18.8 dB, respectively. PTA did not meet improvement criteria in any patient. The serial audiological measurements for each individual patient are available in Supplementary DataSupplementary Data.
Progression-free Survival and Median Time to Progression
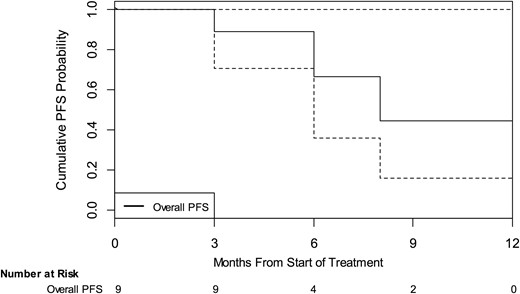
Three patients experienced volumetric progression, and 1 patient experienced hearing progression at a median time of 8 months (95% confidence interval: 3 mo, not yet reached; see Fig. 2).
Kaplan–Meier estimates of cumulative PFS probability. This figure illustrates the overall (black) PFS probability as measured from first dose of study drug to date of progression for all evaluable patients. “Overall PFS” is defined as either volumetric or hearing progression. Dotted lines indicate 95% confidence intervals.
Conclusion
None of our 9 NF2 patients with evaluable disease enrolled on stage 1 of this prospective clinical trial experienced a volumetric or hearing response, and we therefore concluded that everolimus was ineffective for the treatment of progressive VS. We observed the largest volumetric decrease, of 14%, in a cervical nerve root tumor, raising the possibility that everolimus might be more effective in treating non-VS tumors in NF2 patients. Our series, however, did not include other volumetrically measurable nerve root tumors that would have allowed us to address this question.
Comparing our study results with the only other prospective phase II clinical trial for NF2 patients with VS published to date,27 the antitumor activity of everolimus appears inferior to lapatinib. Using the same response criteria, lapatinib performed superiorly, with a 23.5% and a 30.8% volumetric and audiological response rate, respectively.
Everolimus was generally well tolerated in this patient population, with toxicities that were usually mild and manageable. Of note, we did observe grade 3 pulmonary toxicity in 1 patient, necessitating discontinuation of study drug. As many patients with NF2 exhibit lower cranial nerve dysfunction and associated dysphagia, resulting in chronic aspiration and increased risk for aspiration pneumonias, the added risk of everolimus for developing upper and lower respiratory tract infections is of clinical concern in this population.
There are several possible explanations for the lack of observed activity of everolimus in our study. In vitro, inhibition of mTORC1 by rapamycin exerted a selective cytostatic effect but did not induce apoptosis in Merlin-deficient arachnoidal, meningioma, and schwannoma cells.8,9 More recent preclinical data indicate that differential regulation of both mTORC1 and mTORC2 may be cell-type dependent in NF2-deficient tumors and correlate with response to specific classes of mTOR inhibitors.28 Rapamycin and rapalogs, such as everolimus, may release 2 major negative feedback loops that operate to restrain mTORC1 signaling.7 One is mediated by S6K, which phosphorylates and inactivates insulin receptor substrate 1, limiting activation of phosphatidylinositol-3 kinase and Akt.7 The other is mediated by Grb10, which upon phosphorylation by mTORC1 becomes stabilized and binds to receptor tyrosine kinases, opposing their ability to activate both Akt and extracellular signal-regulated kinase.29,30 These feedback loops may be operational in VS in vivo, limiting the efficacy of everolimus. To determine the clinically achievable intratumoral everolimus concentrations in VS tissue and the effects on mTOR signaling, effectors, and feedback inhibition, we are currently conducting a pharmacokinetic/pharmacodynamic (“phase 0”) study of everolimus in presurgical VS and meningioma patients (ClinicalTrials.gov identifier NCT01880749).
In summary, our study indicates that everolimus is generally well tolerated in NF2 patients but does not possess clinically meaningful antitumor activity, within the limitations of our study design. The natural history of tumor growth in NF2 patients is variable,20,31–33 and although it is possible that everolimus has a cytostatic or growth-delaying effect on a subset of NF2-related tumors, conclusive evidence of such an effect would require a much larger, randomized phase II study, which is very difficult to undertake in a rare disease such as NF2. However, 2 other studies of everolimus for NF2 patients with a very similar trial design are currently ongoing (ClinicalTrials.gov identifiers NCT01345136 and NCT01490476) and are expected to provide additional data on the safety, tolerability, and efficacy of everolimus in NF2 patients. Clearly, further studies are needed to direct the future development of molecular targeted therapies aimed at mTORC signaling networks in NF2-associated tumors and improve the treatment of VS in NF2 patients.
Funding
This study was supported in part by the Children's Tumor Foundation (grant 2010-10-011); Novartis Pharmaceuticals, Inc. (study no. CRAD001KUS178T); and the National Institutes of Health (P30 CA016087 to J.D.G., Cancer Center Support Grant to the NYU School of Medicine).
Acknowledgments
We are grateful to Carole W. Mitchell and Erin Hartnett for excellent study-related patient care. This study was presented in part at the 2nd Biennial 2013 Pediatric Neuro-Oncology Basic and Translational Research Conference, Fort Lauderdale, Florida, May 2013, and the Children's Tumor Foundation 2013 NF conference, Monterey, California, June 2013.
Conflict of interest statement. M.A.K. received funding for this study as well as for a separate pharmacokinetic/pharmacodynamic study with everolimus from Novartis Pharmaceuticals under institutional clinical trial agreements. All other authors declare that they have no relevant conflicts of interest.
References
- magnetic resonance imaging
- rapamycin
- adult
- audiometry
- child
- phase 2 clinical trials
- genes, neurofibromatosis 2
- mammals
- neurofibromatosis 2
- neurofibromin 2
- acoustic neuroma
- pediatrics
- neoplasms
- pharmacodynamics
- signal pathway
- everolimus
- tumor growth
- signal transduction pathways
- mtor serine-threonine kinases