-
PDF
- Split View
-
Views
-
Cite
Cite
Soha Talih, Zainab Balhas, Rola Salman, Nareg Karaoghlanian, Alan Shihadeh, “Direct Dripping”: A High-Temperature, High-Formaldehyde Emission Electronic Cigarette Use Method, Nicotine & Tobacco Research, Volume 18, Issue 4, April 2016, Pages 453–459, https://doi.org/10.1093/ntr/ntv080
Close - Share Icon Share
Abstract
Electronic cigarettes (ECIGs) electrically heat and vaporize a liquid solution to produce an inhalable nicotine-containing aerosol. Normally the electrical heater is fed the liquid via an automatic wick system. Some ECIG users, however, elect to directly drip liquid onto an exposed heater coil, reportedly for greater vapor production and throat hit. Use of such “direct drip atomizers” (DDAs) may involve greater exposure to non-nicotine toxicants due to the potentially higher temperatures reached by the coil. In this study we examined nicotine and volatile aldehyde (VA) emissions from one type of DDA under various use scenarios, and measured heater temperature.
Aerosols were machine-generated from an NHALER 510 Atomizer powered by an eGo-T battery (Joyetech), using a common PG-based liquid and a fixed puffing regimen. Inter-drip interval, the number of puffs drawn between replenishing the liquid on the coil, was varied from 2–4 puffs/drip. Total particulate matter, nicotine, and VA yields were quantified. Heater temperature was monitored using an infrared camera.
Depending on the condition, VA emissions, including formaldehyde, greatly exceeded values previously reported for conventional ECIGs and combustible cigarettes, both per puff and per unit of nicotine yield. Increasing the inter-drip interval resulted in greater VA emissions, and lower total particulate matter and nicotine yields. Maximum heater coil temperature ranged from 130°C to more than 350°C.
Due to the higher temperatures attained, DDAs are inherently likely to produce high toxicant emissions. The diversity of ECIG use methods, including potential off-label methods, should be considered as ECIG regulatory efforts proceed.
Introduction
Promoted as a “healthy alternative” to tobacco smoking, electronic cigarette (ECIG) use is rapidly growing in popularity 1–3 , including with tobacco-naïve youth. 4 ECIGs are characterized by a wide and rapidly evolving range of technologies and use methods, 5 and users may choose among numerous basic designs, heating element features, and liquids, yielding thousands of possible combinations. 6 Regardless of the basic design, the operating principle of most if not all ECIGs is similar. When an ECIG user takes a puff, an electric heater coil is activated that vaporizes an “e-liquid,” a propylene glycol (PG) and/or vegetable glycerin (VG) based solution of nicotine and other trace additives. As the vapors pass through the device, they condense to form an aerosol that is inhaled by the user through the mouth-end of the device. Though the mixture exiting the ECIG mouthpiece is commonly referred to by users and many researchers as a “vapor,” it is more correctly termed an “aerosol mist”—a system of liquid droplets suspended in a gas or gas mixture. 7
Basic ECIG designs include products that are a single disposable unit (“cigalikes”), two-piece units with a separate rechargeable battery that screws on to a cartridge (or “cartomizer”) that contains both the liquid and heating element, or three (or more) piece products that consist of a liquid reservoir tank, a heating element, and a battery which all screw together to form a single unit (so-called “tank systems”).
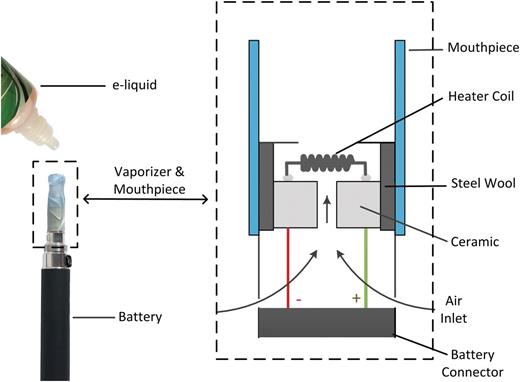
Another form of ECIG use, particularly among experienced ECIG users, is known as “direct dripping.” With this method, every few puffs e-liquid is added directly onto the electrical heating coil of a “direct drip atomizer” (DDA, Figure 1 ). This device provides the user the ability to control the rate of liquid delivery to the heater coil, in contrast to conventional ECIG products which incorporated an automatic wicking mechanism to draw liquid to the heating coil. As a result, users claim, DDAs provide greater vapor yields, stronger throat hit, and more consistent flavor. 8 DDAs are also promoted as offering users a way to test new flavors without the commitment of filling a cartomizer reservoir. 9 While the size of the DDA market is unknown, demand is sufficiently large that many online ECIG vendors sell DDAs as standard ECIG accessories. 10–13
Figure 1. Schematic of electronic cigarette “direct drip atomizer.”
Crucial for experienced tobacco users, and a major concern for public health officials who fear that tobacco-naïve individuals may become addicted, ECIGs have the ability to deliver nicotine, 14 , 15 a key dependence-supporting agent in tobacco smoke. 16 Initial studies on ECIG nicotine delivery found that naïve ECIG smokers attained negligible levels of plasma nicotine when using ECIGs, 17 , 18 while more recent studies found that experienced ECIG users can attain plasma nicotine levels similar to combustible cigarette smokers. 14 These apparently contradictory findings might be explained by the fact that naïve and experienced users puff ECIGs differently, 19–21 and the possibility that these studies utilized ECIG technologies with differing nicotine delivery efficacies. Indeed, when puff topography parameters used in smoking machine studies reflect values typical of experienced ECIG users, nicotine yields are much higher than those obtained when using puff parameters similar to those of naïve ECIG users. 22–24
In addition to nicotine, which is intentionally added to the e-liquid, some toxicants in ECIG aerosols are byproducts of chemical reactions that occur during ECIG use. One example is the class of toxicants known as volatile aldehydes (VA), considered a “major” causative agent in cigarette smoking related pulmonary disease, 25 and which include formaldehyde, an IARC Class 1A carcinogen. Previous studies have found trace levels of VA in ECIG aerosols, 26 , 27 and that VA yields are a function of battery voltage and base liquid composition. 28 , 29 It was also found that the presence of VA in ECIG aerosol increases with increasing puff number, possibly due to rising temperatures as the e-liquid is depleted from the vicinity of the heater coil. 30 Another study analyzed the emissions of 13 ECIG brands, and found large variations in VA yields across brands, and across different samples of the same product. 31 This study also detected the presence of glyoxal and methylglyoxal in the ECIG aerosol. Interestingly, these mutagenic compounds reportedly do not appear in the emissions of combusted tobacco products. 31 , 32
From a toxicant exposure perspective, because DDAs depend on the user to replenish the liquid on the heating coil in a manual, trial-and-error fashion, it is likely that users will inhale aerosols produced under conditions during which the heater coil begins to dry out, and during which relatively high temperatures can be attained which favor the production of VA. Indeed, DDA users are commonly instructed to add more e-liquid whenever a “burned” taste is attained, 33 suggesting onset of pyrolysis chemistry. Adding too much liquid, on the other hand, can result in a “flooded” atomizer where vapor production is suppressed. This makes the DDA an interesting case for the study of VA emissions.
In this study we examined nicotine, total particulate matter (TPM), and gaseous VA emissions from one type of DDA under a variety of use scenarios. In addition, we examined the temperature of the heating coil under various conditions. We hypothesized that DDA use is inherently likely to produce significant VA emissions because the DDA heating coil will attain higher temperatures as the liquid film evaporates between drip events.
Methods
Aerosol Generation and Sampling
A custom-designed digital puff production machine at the American University of Beirut was used to generate ECIG aerosol from one type of DDA (NHALER 510 Atomizer, 2.5 Ohms) powered by an eGo-T battery (3.4V, Joyetech) using a common PG-based liquid (Liquid Express, WaterMelon Chill, 0 or 18mg/mL nicotine concentration). Puff duration was set at 8 seconds, with a puff velocity of 19.1mL/s and an interpuff interval of 10 seconds. These conditions correspond to the “extreme experienced” ECIG user profile previously reported in Talih et al., 24 based on observations by Hua et al. 19
The aerosol was drawn from the mouth end of the DDA, through a glass fiber filter (Gelman Type A/E 47mm) followed by a DNPH-coated silica cartridge ( Supplementary Figure S1 ). The filter was analyzed for nicotine content by gas chromatography-mass spectrometry, while the DNPH cartridge was analyzed by high-performance liquid chromatography-mass spectrometry for derivatized VA species. 24
The e-liquid dripping regimen was chosen to model that of DDA users. We found in a convenience sample of YouTube videos and ECIG forum postings that DDA users commonly draw puffs until they experience a “burned taste,” at which point they add a few drops, and resume puffing. The number of puffs drawn between dripping (the “interdrip interval” [IDI]) was most commonly 3–6 puffs. 34 To account for varying dripping behavior, we selected three experimental conditions in which IDI was specified as 2, 3, or 4 puffs; that is, either 2, 3, or 4 puffs were executed for every two drops (27.3±0.66mg/drop) of e-liquid added to the DDA. For each experimental condition, a minimum of three sets of samples were generated for nicotine and aldehyde determinations. Each sample was generated by combining the vapors emitted from the specified number of puffs per drip (ie, 2, 3, or 4 puffs) from each of three DDAs of the make and model specified above. The results were then extrapolated to 15 puffs for comparison across conditions.
Because the DDAs used in this study arrive bone-dry from the manufacturer, initial applications of liquid to the coil are quickly absorbed by the steel mesh of the device (Figure 1), leaving the coil relatively dry and aerosol production weak. As the mesh becomes saturated after repeated use, most of the e-liquid dripped onto the coil remains on the coil, and steady, repeatable aerosol production can be attained. Thus it is critical to prime each DDA device prior to commencing measurements. The priming procedure consisted of adding 4–5 drops of e-liquid onto the DDA, executing multiple puffs, and measuring the residual mass of the DDA (ie, the amount of liquid remaining in the DDA, assessed gravimetrically). This process was repeated until the residual mass of e-liquid in the DDA reached a steady value, approximately 70mg. At this point the device was considered primed and tests with the DDA could commence.
Chemical Analysis
TPM was determined gravimetrically by weighing the filter pad and holder before and after each sampling session. In addition, the entire DDA assembly, drip tip, connection tubing were also weighed before and after each test session to allow for a mass balance accounting of the e-liquid.
Filters were then extracted and analyzed by gas chromatography-mass spectrometry (Thermo Scientific TRACE GC-Ion Trap MS, Thermo Fisher Scientific, Waltham, MA) for nicotine content in accordance with previously described methods. 35 , 36 Percent recovery of nicotine standards were calculated by spiking glass fiber filters with nicotine standard at concentrations ranging from 5 to 75 μg/ml. Standards were extracted and analyzed using the same procedure reported in. 35 , 36 This procedure results in an average recovery near 90%, and a limit of detection of 0.5 μg.
Volatile aldehydes were determined by extracting the DNPH-coated cartridges in acetonitrile (ACN) and analyzing the solution by high-performance liquid chromatography-mass spectrometry (LC/MSD Trap XCT, Agilent Technologies, Santa Clara, CA). 37 Cartridges were stored at 4°C until analysis, within 48 hours of sampling. The species analyzed, and the limit of detection and limit of quantitation were respectively as follows (µg): formaldehyde, 0.07 and 0.23; acetaldehyde, 0.11 and 0.37; acetone, 0.13 and 0.42; acrolein, 0.2 and 0.68; propionaldehyde, 0.06 and 0.19; crotonaldehyde, 0.14 and 0.47; methacrolein, 0.001 and 0.003; butyraldehyde, 0.05 and 0.16; and valeraldehyde, 0.09 and 0.32. A representative high-performance liquid chromatography chromatogram is shown in Supplementary Figure S2 .
Temperature Measurements
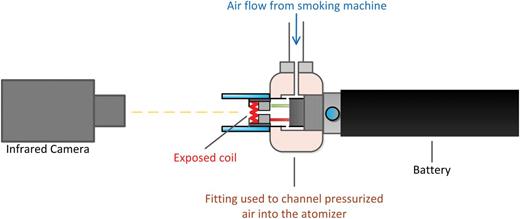
In separate experiments, heater coil temperatures were measured using an infrared camera (ThermaCAM 560, FLIR Systems, Wilsonville, OR) and a home-built reverse-puffing apparatus which allowed the mouthpiece view to remain unobstructed for the infrared camera ( Figure 2 ). Puffs were generated by forcing air through the inlet port of the atomizer, rather than drawing air under vacuum through the outlet port (ie, the mouthpiece). The pressure was controlled in such a manner that the reverse puffs were executed at precisely the same flow rate and interpuff interval as the normal puffing procedure, resulting in identical flow conditions within the DDA. The infrared camera was positioned facing the exposed heater coil at a 90cm distance, the nearest distance attainable given the focal length of the camera lens. The peak measured temperature of the coil surface was recorded in a 0.5 second time increments for four consecutive puffs.
Experimental set-up for measuring the electronic cigarette (direct drip atomizer) heater coil maximum temperature.
Statistical Analysis
Outcome variables including TPM, nicotine, and volatile aldehydes were summarized as mean ± standard deviation. Dependent (outcome) variables were arranged and compared based on IDI using one-way analysis of variance including post-hoc pairwise comparisons (Bonferroni). P < .05 was used to indicate statistical significance. Statistical analyses were done using SPSS version 21.0 (IBM, Armonk, NY).
For a valid comparison across conditions, TPM, nicotine and volatile aldehyde yields were extrapolated to 15 puffs. Because puff volume was constant across conditions, the reported yields also correspond to the same total aerosol volume.
Results
A summary of findings is presented in Table 1 . Values of nicotine yields ranged from 0.62 to 2.95mg, while total aldehydes ranged from 50 to more than 2000mg per 15 puffs. Mass balance analysis of the dataset showed that all of the liquid that evaporated from the DDA heater coil could be quantitatively traced. Forty-nine percent of the mass evaporating from the DDA recondensed on the DDA mouthpiece, 2% condensed on the tubing connecting the mouthpiece to the filter sampler, and 49% was collected on the filter pad. Similar results were attained for all experimental conditions.
Measured Nicotine and VA Yields in 15 Puffs (Mean ± SD ; n = 3)
| Device . | DDA (present study) . | Combustible cigarette—3R4F 38,39 . | “Cigalike” ECIG 22,27 . | “Tank” ECIG 28 . | ||
|---|---|---|---|---|---|---|
| Two puffs IDI . | Three puffs IDI . | Four puffs IDI . | ||||
| Nicotine (mg) | 1.03±0.061* | 0.91±0.12 | 0.74±0.05 | 0.91 | 0.025–0.77 | NR |
| TPM (mg) | 186.6±11.60 | 176.33±20.94 | 147.46±15.58* | NR | NR | NR |
| VA yields (μg) | ||||||
| Formaldehyde | 19.70±15.95* | 71.30±9.22 | 88.06±9.43 | 21.5±7.8 | 0.20–5.61 | 0.02–27 a |
| Acetaldehyde | 269.35±182.28* | 822.70±32.05 | 1172.23±87.04* | 540.3±135.3 | 0.11–1.36 | 0.17–4.23 a |
| Acetone | 28.28±20.66 | 103.28±55.42 | 196.55±49.91 | 214.1±43.4 | NR | 0.34–7.59 a |
| Acrolein | BDL | 1.97±3.36 | 1.75±0.71 | 49.0±14.1 | 0.07–4.19 | NR |
| Propionaldehyde | 51.58±35.86* | 184.98±15.39 | 314.54±32.58* | 42.6±8.0 | NR | ND b |
| Crotonaldehyde | BQL | BQL | BQL | 13.2±5.2 | NR | BQL b |
| Methacrolein | BQL | 0.85±0.25 | 0.95±0.44 | NR | NR | NR |
| Butyraldehyde | 0.61±0.98* | 2.98±0.60 | 6.30±0.36* | 26.7±6.2 | NR | NR |
| Valeraldehyde | 29.12±26.68* | 83.90±12.09 | 92.49±10.28 | NR | NR | BQL b |
| Total aldehydes | 398.63±280.54* | 1271.97±41.72 | 1872.86±154.25* | NR | NR | NR |
| Device . | DDA (present study) . | Combustible cigarette—3R4F 38,39 . | “Cigalike” ECIG 22,27 . | “Tank” ECIG 28 . | ||
|---|---|---|---|---|---|---|
| Two puffs IDI . | Three puffs IDI . | Four puffs IDI . | ||||
| Nicotine (mg) | 1.03±0.061* | 0.91±0.12 | 0.74±0.05 | 0.91 | 0.025–0.77 | NR |
| TPM (mg) | 186.6±11.60 | 176.33±20.94 | 147.46±15.58* | NR | NR | NR |
| VA yields (μg) | ||||||
| Formaldehyde | 19.70±15.95* | 71.30±9.22 | 88.06±9.43 | 21.5±7.8 | 0.20–5.61 | 0.02–27 a |
| Acetaldehyde | 269.35±182.28* | 822.70±32.05 | 1172.23±87.04* | 540.3±135.3 | 0.11–1.36 | 0.17–4.23 a |
| Acetone | 28.28±20.66 | 103.28±55.42 | 196.55±49.91 | 214.1±43.4 | NR | 0.34–7.59 a |
| Acrolein | BDL | 1.97±3.36 | 1.75±0.71 | 49.0±14.1 | 0.07–4.19 | NR |
| Propionaldehyde | 51.58±35.86* | 184.98±15.39 | 314.54±32.58* | 42.6±8.0 | NR | ND b |
| Crotonaldehyde | BQL | BQL | BQL | 13.2±5.2 | NR | BQL b |
| Methacrolein | BQL | 0.85±0.25 | 0.95±0.44 | NR | NR | NR |
| Butyraldehyde | 0.61±0.98* | 2.98±0.60 | 6.30±0.36* | 26.7±6.2 | NR | NR |
| Valeraldehyde | 29.12±26.68* | 83.90±12.09 | 92.49±10.28 | NR | NR | BQL b |
| Total aldehydes | 398.63±280.54* | 1271.97±41.72 | 1872.86±154.25* | NR | NR | NR |
BDL = below detectable limit; BQL = below quantifiable limit; DDA = direct drip atomizers; ECIG = electronic cigarette; IDI = interdrip interval; NR = not reported; TPM = total particulate matter; VA = volatile aldehyde.
a Values reported for two voltages.
b Values reported for one voltage
Measured Nicotine and VA Yields in 15 Puffs (Mean ± SD ; n = 3)
| Device . | DDA (present study) . | Combustible cigarette—3R4F 38,39 . | “Cigalike” ECIG 22,27 . | “Tank” ECIG 28 . | ||
|---|---|---|---|---|---|---|
| Two puffs IDI . | Three puffs IDI . | Four puffs IDI . | ||||
| Nicotine (mg) | 1.03±0.061* | 0.91±0.12 | 0.74±0.05 | 0.91 | 0.025–0.77 | NR |
| TPM (mg) | 186.6±11.60 | 176.33±20.94 | 147.46±15.58* | NR | NR | NR |
| VA yields (μg) | ||||||
| Formaldehyde | 19.70±15.95* | 71.30±9.22 | 88.06±9.43 | 21.5±7.8 | 0.20–5.61 | 0.02–27 a |
| Acetaldehyde | 269.35±182.28* | 822.70±32.05 | 1172.23±87.04* | 540.3±135.3 | 0.11–1.36 | 0.17–4.23 a |
| Acetone | 28.28±20.66 | 103.28±55.42 | 196.55±49.91 | 214.1±43.4 | NR | 0.34–7.59 a |
| Acrolein | BDL | 1.97±3.36 | 1.75±0.71 | 49.0±14.1 | 0.07–4.19 | NR |
| Propionaldehyde | 51.58±35.86* | 184.98±15.39 | 314.54±32.58* | 42.6±8.0 | NR | ND b |
| Crotonaldehyde | BQL | BQL | BQL | 13.2±5.2 | NR | BQL b |
| Methacrolein | BQL | 0.85±0.25 | 0.95±0.44 | NR | NR | NR |
| Butyraldehyde | 0.61±0.98* | 2.98±0.60 | 6.30±0.36* | 26.7±6.2 | NR | NR |
| Valeraldehyde | 29.12±26.68* | 83.90±12.09 | 92.49±10.28 | NR | NR | BQL b |
| Total aldehydes | 398.63±280.54* | 1271.97±41.72 | 1872.86±154.25* | NR | NR | NR |
| Device . | DDA (present study) . | Combustible cigarette—3R4F 38,39 . | “Cigalike” ECIG 22,27 . | “Tank” ECIG 28 . | ||
|---|---|---|---|---|---|---|
| Two puffs IDI . | Three puffs IDI . | Four puffs IDI . | ||||
| Nicotine (mg) | 1.03±0.061* | 0.91±0.12 | 0.74±0.05 | 0.91 | 0.025–0.77 | NR |
| TPM (mg) | 186.6±11.60 | 176.33±20.94 | 147.46±15.58* | NR | NR | NR |
| VA yields (μg) | ||||||
| Formaldehyde | 19.70±15.95* | 71.30±9.22 | 88.06±9.43 | 21.5±7.8 | 0.20–5.61 | 0.02–27 a |
| Acetaldehyde | 269.35±182.28* | 822.70±32.05 | 1172.23±87.04* | 540.3±135.3 | 0.11–1.36 | 0.17–4.23 a |
| Acetone | 28.28±20.66 | 103.28±55.42 | 196.55±49.91 | 214.1±43.4 | NR | 0.34–7.59 a |
| Acrolein | BDL | 1.97±3.36 | 1.75±0.71 | 49.0±14.1 | 0.07–4.19 | NR |
| Propionaldehyde | 51.58±35.86* | 184.98±15.39 | 314.54±32.58* | 42.6±8.0 | NR | ND b |
| Crotonaldehyde | BQL | BQL | BQL | 13.2±5.2 | NR | BQL b |
| Methacrolein | BQL | 0.85±0.25 | 0.95±0.44 | NR | NR | NR |
| Butyraldehyde | 0.61±0.98* | 2.98±0.60 | 6.30±0.36* | 26.7±6.2 | NR | NR |
| Valeraldehyde | 29.12±26.68* | 83.90±12.09 | 92.49±10.28 | NR | NR | BQL b |
| Total aldehydes | 398.63±280.54* | 1271.97±41.72 | 1872.86±154.25* | NR | NR | NR |
BDL = below detectable limit; BQL = below quantifiable limit; DDA = direct drip atomizers; ECIG = electronic cigarette; IDI = interdrip interval; NR = not reported; TPM = total particulate matter; VA = volatile aldehyde.
a Values reported for two voltages.
b Values reported for one voltage
Among the compounds tested, formaldehyde, acetaldehyde, acetone, propionaldehyde, and valeraldehydes were quantified. Acrolein, methacrolein, and butyraldehyde were detected but were present in concentrations below limit of quantitation. Crotonaldehyde was not detected.
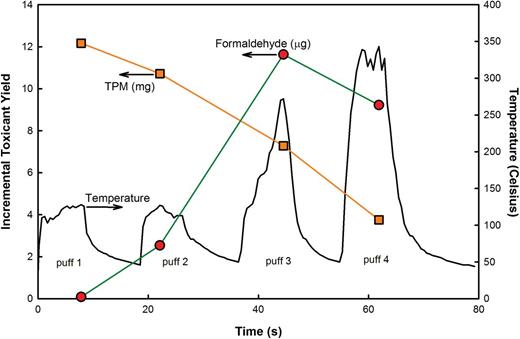
The results of a post-hoc analysis are indicated in Table 1 . In general, increasing the IDI resulted in lower quantities of TPM and nicotine yield and greater quantities of VA emissions. This finding is illustrated in Figure 3 which shows the average incremental yields of TPM and formaldehyde for each consecutive puff. This behavior is further explained in the discussion section below.
Typical maximum heater coil temperature vs. time for four consecutive puffs (8 seconds puff duration, 19.1mL/s puff velocity). Average incremental yields of total particulate matter and formaldehyde are also shown for each consecutive puff.
Typical maximum heater coil temperatures during four consecutive 8-second puffs are presented in Figure 3 . For all measurement sets, it was found that the maximum coil temperatures increased systematically between the second and fourth puff, reaching a peak temperature of greater than 340°C during puff 4.
Discussion
The primary aim of this study was to learn whether “dripping” e-liquids directly onto a heater coil can produce significant levels of non-nicotine toxicant emissions. We chose to examine VA because this class of toxicants can be formed through pyrolysis of oxygenated organics such as PG and VG at elevated temperatures. Given the poorly documented use behavior of ECIGs in general, and of DDA use in particular, we examined VA emissions under several possible use scenarios, focusing on how often the user replenishes the heater coil with fresh e-liquid. It was hypothesized that this variable is critical to VA yields, since, with more puffing between liquid replenishment, the heater coil is more likely to dry out and its temperature will rise significantly, activating VA formation chemistry. Conversely, maintaining a liquid film around the electrical coil by often replenishing the fluid should suppress VA formation and emissions.
The puff-resolved data shown in Figure 3 are entirely consistent with this picture. During the first two puffs, aerosol production is high (TPM > 10mg/puff), indicating ample e-liquid available for vaporization by the coil, and peak temperatures do not exceed 130°C. Formaldehyde emissions remain similar to those of conventional combustible cigarettes (20–50 µg/mg nicotine). During puff 3, the coil temperature rises above 250°C, and the TPM production drops to about half that of puff 1, while during puff 4 the temperature rises further still, and the TPM production drops further to approximately one-third that of puff 1. Simultaneously, the formaldehyde emissions rise with each passing puff. Thus depending on the IDI adopted by the user, a 10–15 puff bout will involve one to multiple combustible cigarette equivalents of formaldehyde (and other VAs; see Table 1 ). While scientific observation of DDA user practices has not been reported, online primers for novice DDA users instruct users to add more liquid when a “burned taste” appears, 40 in all likelihood indicating the onset of the high temperature, high VA emissions operating regime seen in puffs 3 and 4. It is noteworthy that even if DDA users drip in a manner that avoids these high temperature conditions, this study indicates that they would nonetheless attain VA levels comparable to those of combustible cigarettes. Taken together, the data provide strong indication that DDA use likely exposes users to combustible cigarette-like and greater levels of VA, when used to obtain cigarette-like levels of nicotine.
This finding highlights the importance for regulators to consider the diversity of ECIG technologies and use methods, and to address potential off-label uses of ECIG products. This study also highlights the importance of ECIG analytical lab methods to be based on observations of human behavior, since the results are strongly dependent on the puffing/dripping regimen used.
Limitations of this study include reliance on only one intensive puff topography regimen for generating the aerosols. As has been previously shown puff topography variables can significantly alter toxicant yields. 24 Moreover, device power and PG/VG ratios have been previously shown to affect the levels of ECIG VA emissions, 28 and these variables were not examined in this study. While a larger study with a wider range of variables would elucidate the plausible ranges of VA yields from DDAs under various use conditions, the current study is sufficient to highlight an inherent drawback of inhaling ECIG aerosols generated by a manual liquid application process.
An additional limitation stems from the fact that VA species were not measured in the liquid prior to vaporization, 41 and therefore some portion of the measured VA yields may have been present at the outset. We also note that while only gaseous VAs were analyzed in this study, there may be significant quantities of VAs also found in the particle phase of the ECIG aerosol that was trapped on the sampling filter pad ( Supplementary Figure S1 ), as recently found by Jensen et al. 29
Conclusion
Depending on operating characteristics and use protocol, VA emissions from ECIG devices range from negligible amounts, 26 , 27 to levels similar to those present in tobacco cigarette smoke. 28 , 30 Dripping, apart from its clear implications for drug abuse liability, may also involve greater exposure to VA due to the potentially higher temperatures attained in the atomizer. Based on our results, DDA use may expose users to increased VA levels relative to conventional ECIGs and even relative to combustible cigarettes, for a given nicotine yield.
Funding
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (grant number P50DA036105) and the Center for Tobacco Products of the US Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Declaration of Interests
None declared.
References
Shihadeh, A., & Eissenberg, T. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux.
Hinds W. Aerosol technology: Properties, behavior, and measurement of airborne particles.
Dripping atomizers of the disposable kind . 2014. http://spinfuel.com/dda-disposable-dripping-atomizers/ . Accessed November 28, 2014.
GREENPUFF. 510 bridgeless dripping atomizer. http://www.greenpuffinc.com/electronic-cigarette-accessories/510-bridgeless-dripping-atomizers.html . 2011. Accessed December 1, 2014.
USDHHS, ed. The Health Consequences of Smoking—50 Years of Progress: Nicotine Addiction: A Report of the Surgeon General . Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, U.S. Department of Health and Human Services; 2014. ww.surgeongeneral.gov/library/reports/50-years-of-progress/index.html#fullreport . Accessed April 16, 2015.
Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos.
Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects.
Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes.
Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities.
Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, . . . Shihadeh A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions.
Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, . . . Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes.
Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage.
ModernVapor. Direct dripping—A quicker way to fill your E-cig with juice. 2011. http://www.blog.modernvapor.com/e-cig-technology/direct-dripping-%E2%80%93-a-quicker-way-to-fill-your-e-cig-with-juice.htm . Accessed April 16, 2014.
E-cigarette forum. 2010. www.e-cigarette-forum.com/forum/new-membersforum/119891-how-many-puffs-per-drip.html . Accessed December 1, 2014.
Al Rashidi M, Shihadeh A, Saliba NA. Volatile aldehydes in the mainstream smoke of the narghile waterpipe.
Intorp M, Purkis S, Wagstaff W. Determination of carbonyl compounds in cigarette mainstream smoke. the CORESTA 2010 collaborative study and recommended method.
Beginner’s guide to the art of dripping. 2014. http://vaporcloudreviews.com/ beginners-guide-to-dripping/ . Accessed December 1, 2014.




Comments