-
PDF
- Split View
-
Views
-
Cite
Cite
Wilfredo R Matias, Jacob M Koshy, Ellen H Nagami, Victor Kovac, Letumile R Moeng, Erica S Shenoy, David C Hooper, Lawrence C Madoff, Miriam B Barshak, Jennifer A Johnson, Christopher F Rowley, Boris Julg, Elizabeth L Hohmann, Jacob E Lazarus, Tecovirimat for the Treatment of Human Monkeypox: An Initial Series From Massachusetts, United States, Open Forum Infectious Diseases, Volume 9, Issue 8, August 2022, ofac377, https://doi.org/10.1093/ofid/ofac377
Close - Share Icon Share
Abstract
A large, ongoing multicountry outbreak of human monkeypox has the potential to cause considerable morbidity and mortality. Therapeutics for the treatment of smallpox, a related Orthopoxvirus, may be used and affect the natural history of monkeypox. We present 3 patients from our hospitals treated with tecovirimat, a pan-Orthopoxvirus inhibitor currently available under an expanded access investigational new drug protocol for monkeypox.
Monkeypox virus (MPXV) is an Orthopoxvirus endemic to Central and West Africa with a presumed rodent reservoir [1]. Over the past 30 years, zoonotic transmissions have increased, likely due to progressive environmental encroachment as well as waning population-level immunity to the related Variola virus (smallpox) and Vaccinia virus (smallpox vaccine) [2]. Previous outbreaks outside the African continent have been rare and person-to-person transmission has been limited [3–5].
In May 2022, a large outbreak caused by clade 3 (“West African”) virus was detected in multiple nonendemic countries [6–8]. Sustained person-to-person transmission has resulted in thousands of cases [9]. “Central African” clade virus may have greater inherent pathogenicity compared to West African clade virus; however, mortality and serious morbidity have resulted from West African clade virus in children, pregnant women, and immunocompromised individuals, demonstrating the need for effective medical countermeasures [8, 10, 11]. Here we describe the first 3 cases in Massachusetts treated with tecovirimat, a small molecule Orthopoxvirus inhibitor that is US Food and Drug Administration (FDA)–approved for the treatment of smallpox, and for which the Centers for Disease Control and Prevention (CDC) holds an expanded access investigational new drug protocol for monkeypox [12].
Case 1
A man in his 20s with a history of gonococcal urethritis developed a subjective fever while on an international trip (to a nonendemic country that has been affected by the current outbreak). While there, he had unprotected sex with men. One week later, he presented to outpatient care in the US with subjective chills and malaise and a mildly painful, shallow ulcer on the foreskin of the penis. He was treated empirically for syphilis. Testing for syphilis, gonorrhea, and chlamydia was negative. The ulcer enlarged, and he developed new ulcers over the pubis and painful left inguinal lymphadenopathy. He returned to care and was treated empirically for herpes simplex virus (HSV) and chancroid. An HSV culture returned negative. Over the following 3 days he developed painful, pruritic vesiculopustular lesions on the face, oropharynx, hands, and feet (including the soles), prompting presentation to the hospital.
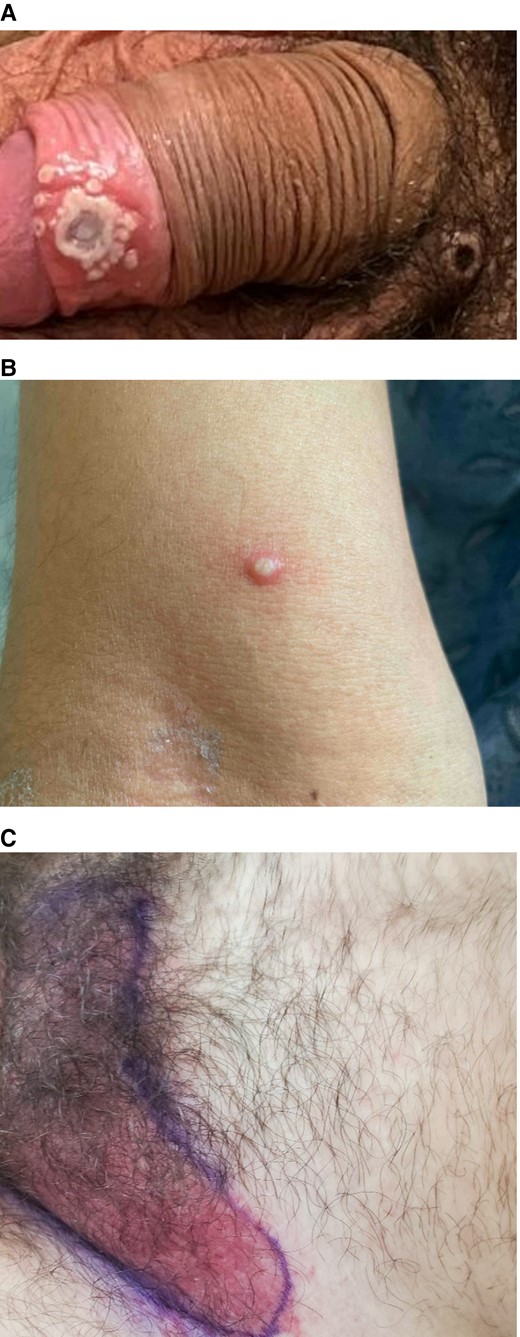
Material obtained from an oropharyngeal, a penile (Figure 1A), and a facial pustule was sent to the Massachusetts Department of Public Health (MDPH) where polymerase chain reaction (PCR) was positive for Orthopoxvirus. Subsequent testing at CDC confirmed MPXV clade 3 (West African clade, consistent with the current outbreak). He was admitted to the hospital in the setting of inability to isolate at home. On hospital day 2, tecovirimat at a dose of 600 mg twice daily was initiated orally due to painful genital lesions. By day 4 of tecovirimat, development of new lesions had ceased and the patient noted a decrease in pain and pruritus, and several of the more recent lesions decreased in size, stopped expanding, or resolved without progress from vesicles to pustules. On day 6 of tecovirimat, alanine aminotransferase (ALT) rose from 31 U/L to 72 U/L (upper limit of normal, 55 U/L), before falling to 57 U/L the following day and normalizing spontaneously by day 8 of tecovirimat. The patient was on no other systemic medications on the 2 days prior to the rise in the ALT. On hospital day 10 (day 9 of tecovirimat), he was transferred to another facility to complete his isolation. By day 14 of tecovirimat (end of therapy), he reported resolution of the majority of his lesions. His only reported adverse effect on tecovirimat was a mild nonfocal headache associated with the first dose.
Cropped clinical images of case patients treated with tecovirimat. We highlight here the diverse clinical manifestations in our patients. A, Vesiculopustular lesion with surrounding satellite pustules on the foreskin of Case 1. Note additional scabbed lesion on the pubis. B, A pustular lesion on the arm of Case 2. C, The erythematous perineal rash that prompted administration of broad-spectrum antibacterial for Case 3. Not pictured is the vesicular component.
Case 2
A man in his 20s with human immunodeficiency virus (HIV) (on antiretroviral therapy with a suppressed viral load and CD4 count >500 cells/µL) presented to outpatient care 7 days after anal-receptive intercourse with a person subsequently confirmed to have monkeypox infection. He received 1 dose of a replication-deficient modified vaccinia Ankara vaccine (JYNNEOS) in the outpatient setting as postexposure prophylaxis, but the following day, he developed subjective fevers and chills, myalgias, and left tonsillar pain with associated odynophagia. Two days later the development of scattered erythematous pustules on his forearms and hands prompted presentation to the hospital.
There, he was febrile to 39.5°C and was admitted to the hospital. Computed tomography of the neck demonstrated extensive bilateral cervical lymphadenopathy and a prominent left palatine tonsil without edema. Material from a pustular lesion on his right arm (Figure 1B) was PCR positive for Orthopoxvirus at MDPH and later confirmed as MPXV clade 3 at CDC. His fever resolved spontaneously within 24 hours of presentation. Treatment with tecovirimat at a dose of 600 mg twice daily was initiated orally on hospital day 2 due to fever and odynophagia with associated difficulty taking solid food. He subsequently developed additional pustular lesions on his gingiva (on day 2 of therapy) and upper and lower extremities (on day 3 of therapy). Tonsillar edema and odynophagia improved slowly after initiation of tecovirimat, and he was discharged on day 5 of therapy (day 7 of hospitalization) once able to comfortably swallow food and medications. At an outpatient visit on day 9 of therapy, the patient reported that all skin lesions had crusted. No biochemical abnormalities were noted while in the hospital. The only reported adverse effect on tecovirimat was 1–2 loose bowel movements a few hours after each dose. He was planned to complete 14 days of therapy.
Case 3
A man in his 40s on preexposure prophylaxis for HIV presented to outpatient care with 1 day of malaise, subjective fevers, and a maculopapular rash involving the perineum. He also noted several vesicles, most noticeably on the foreskin of his penis. He reported unprotected sexual intercourse with multiple men approximately 8–10 days prior while on an international trip (to a nonendemic country that has been affected by the current outbreak). Three days later, progression of his rash prompted presentation to the hospital.
There, he was found to have a confluent dusky, erythematous rash of the perineum with numerous umbilicated pustules on the foreskin of his penis as well as his chest and arm. A lesion was also noted on his right lower eyelid without obvious corneal involvement. He was admitted to the hospital for care of his cellulitis and for ophthalmology evaluation. Material from a ruptured vesicle obtained at his initial outpatient visit had previously returned PCR positive for Orthopoxvirus at MDPH and was later confirmed as MPXV clade 3 at CDC. Due to the erythematous nature of his rash (Figure 1C), intravenous broad-spectrum antibiotics were begun for possible superimposed bacterial cellulitis. His rash did not improve on antibacterials, so on hospital day 2, treatment with tecovirimat 600 mg orally twice daily was initiated. By day 2 of tecovirimat, the perineal rash had become less erythematous and many of his pustular lesions had begun to resolve (without progressing first to crusts). The patient was subsequently discharged on hospital day 4 to complete 14 days of tecovirimat. When seen in outpatient follow-up on day 7 of tecovirimat, he was noted to have near-complete resolution of his rash as well as marked improvement in his right eyelid lesion. No biochemical abnormalities were noted while in the hospital. No side effects associated with tecovirimat were reported.
DISCUSSION
Tecovirimat was identified by a high-throughput screen conducted for compounds with inhibitory in vitro activity against Vaccinia virus and Cowpox virus. It appears to act by inhibiting the product of the F13L gene, which is conserved throughout orthopoxviruses [12]. Pivotal studies subsequently demonstrated protection from mortality in a Rabbitpox virus model of smallpox in rabbits, as well as a Monkeypox virus model of smallpox in nonhuman primates [13]. Pharmacokinetic and safety studies of 361 healthy controls randomized to 600 mg tecovirimat orally twice daily achieved levels 4 times that associated with efficacy in nonhuman primates. No safety signals were identified, and adverse effects were similar to placebo [13]. Tecovirimat is currently available from the United States Strategic National Stockpile and can be administered under the careful monitoring specified under the CDC Institutional Review Board protocol. The CDC currently advises consideration of tecovirimat in patients with severe disease, those at risk for severe disease, and those with disease involvement in anatomic areas that might constitute a special hazard (such as the genitals) [14].
Data on efficacy of tecovirimat against MPXV in humans are limited. A secondary case (from a returning traveler from Nigeria in 2021) was observed to have an increase in viral DNA PCR cycle threshold from the oropharynx and blood coincident with tecovirimat initiation. No new lesions developed after 24 hours of tecovirimat. No adverse effects were reported and no hematological or biochemical abnormalities were noted [3]. Detailed clinical information is not reported for 2 other treated patients (1 in 2021 returning from Nigeria and 1 involved in the current outbreak) [6, 15].
Consistent with human safety studies, we did not observe prominent side effects in this small series. A mild increase in ALT in 1 patient resolved without tecovirimat discontinuation. Our patients had improvement of their lesions on therapy and none progressed to severe disease while on tecovirimat, though given the inherent limitations of this small case series, we cannot speak confidently to its effectiveness. Importantly, our patients were all admitted to the hospital. Since most patients may not require hospitalization and will not initiate tecovirimat under direct supervision, further research should focus on outpatient treatment as well.
Alternative treatments are under investigation. Brincidofovir, a nucleotide analogue FDA-approved for the treatment of smallpox, has demonstrated efficacy against MPXV in rodent models [16, 17]. Brincidofovir has decreased nephrotoxicity compared to its parent compound, cidofovir [18], although all 3 patients treated with brincidofovir in the Liverpool outbreak discontinued therapy due to liver biochemical derangements [3].
Ultimately, given the potential for morbidity and mortality in the current outbreak, prospective, large, well-controlled studies that are powered to demonstrate efficacy are urgently needed. Particular attention should be paid to any ability to accelerate healing of lesions, as an agent with the potential to reduce the duration of infectivity would be particularly desirable.
Notes
Acknowledgments. We thank our patients for consenting to publication of this report and the staff of our respective microbiology laboratories for their crucial partnership. Drs Sandra Smole, Glen Gallagher, Catherine Brown, and Dylan Tierney from the Massachusetts Department of Public Health speedily and ably facilitated diagnosis and treatment of our patients.
Patient consent. The patients provided written informed consent for publication of this work. This study has been determined by the Mass General Brigham Institutional Review Board to not constitute human subjects research.
Financial support. This work was supported by the National Institutes of Health (award numbers T32 AI007433 to W. R. M. and K08 AI155830 to J. E. L.).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Potential conflicts of interest. All authors: No reported conflicts of interest.





Comments