-
PDF
-
Views
-
Cite
Cite
Mona Hussein, Wael Fathy, Ragaey A Eid, Hoda M Abdel-Hamid, Ahmed Yehia, Mostafa S Sheemy, Christine Ragaie, Ahmed Dahshan, Hoda I Rizk, Nabila laz, Rehab Magdy, Relative Frequency and Risk Factors of COVID-19 Related Headache in a Sample of Egyptian Population: A Hospital-Based Study, Pain Medicine, Volume 22, Issue 9, September 2021, Pages 2092–2099, https://doi.org/10.1093/pm/pnab020
Close - Share Icon Share
Abstract
Headache is considered one of the most frequent neurological manifestations of coronavirus disease 2019 (COVID-19). This work aimed to identify the relative frequency of COVID-19-related headache and to clarify the impact of clinical, laboratory findings of COVID-19 infection on headache occurrence and its response to analgesics.
Cross-sectional study.
Recovered COVID-19 patients.
In total, 782 patients with a confirmed diagnosis of COVID-19 infection.
Clinical, laboratory, and imaging data were obtained from the hospital medical records. Regarding patients who developed COVID-19 related headache, a trained neurologist performed an analysis of headache and its response to analgesics.
The relative frequency of COVID-19 related headache among our sample was 55.1% with 95% confidence interval (CI) (.516–.586) for the estimated population prevalence. Female gender, malignancy, primary headache, fever, dehydration, lower levels of hemoglobin and platelets and higher levels of neutrophil/lymphocyte ratio (NLR) and CRP were significantly associated with COVID-19 related headache. Multivariate analysis revealed that female gender, fever, dehydration, primary headache, high NLR, and decreased platelet count were independent predictors of headache occurrence. By evaluating headache response to analgesics, old age, diabetes, hypertension, primary headache, severe COVID-19, steroid intake, higher CRP and ferritin and lower hemoglobin levels were associated with poor response to analgesics. Multivariate analysis revealed that primary headache, steroids intake, moderate and severe COVID-19 were independent predictors of non-response to analgesics.
Headache occurs in 55.1% of patients with COVID-19. Female gender, fever, dehydration, primary headache, high NLR, and decreased platelet count are considered independent predictors of COVID-19 related headache.
Introduction
Coronavirus disease 2019 (COVID-19) has rapidly spread around the world after its first appearance in the Chinese city of Wuhan in December 2019. Because of its fast spread and rapidly growing impact, the coronavirus outbreak is now considered a global health crisis affecting more than 40 million people worldwide [1]. There is a great diversity of symptoms caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Nevertheless, some patients are asymptomatic. In general, the clinical manifestations of COVID-19 infection are nonspecific, they include fever, bony pains, shortness of breath, cough, and gastrointestinal symptoms [2, 3].
Headache is a commonly described symptom in patients with COVID-19 [4]. There is a wide variation in the estimated prevalence of headache in patients with COVID-19 infection between Chinese and European studies. While a meta-analysis of Chinese studies estimated headache prevalence at 12%, European studies found that headache prevalence increased to be 70% [5, 6]. Yet there are no data available on the prevalence of headaches among Egyptians or even the Arab population.
It is well known that the SARS-CoV-2 inspires inflammatory cascades, which trigger a cytokine storm, resulting in an alteration in immune response in the form of leucopenia and lymphopenia. Elevated serum levels of C-reactive protein (CRP), ferritin, or D-dimer were also reported in some patients [7].
Whether the COVID-19 related headache is related to the COVID severity, to some inflammatory markers, or some other symptoms such as fever or dehydration, it is still a matter of debate [8, 9].
This work aimed to determine the relative frequency of COVID-19 related headache in a sample of the Egyptian population. It also aimed to study the impact of the clinical characteristics, laboratory data, and severity of COVID-19 infection on the occurrence of COVID-19 related headache and its response to analgesics.
Methods
Study design:This cross-sectional study was carried out on 782 patients who were diagnosed with COVID-19 infection between April 1 and June 30, 2020 and were admitted to two government-authorized centers to treat COVID-19 patients (Beni-Suef University Hospital and Beni-Suef Insurance Hospital). By referring to the hospital records and after extracting the phone numbers of COVID-19 patients who were admitted during the above-mentioned period, participants were recruited by systematic random sampling and phoned.
Eligibility criteria:Patients with a confirmed diagnosis of SARS-CoV-2 infection, according to interim guidelines of the World Health Organization (WHO) [10], were included. The nasopharyngeal swab results were tested positive by real-time reverse transcription polymerase chain reaction (RT-PCR) assay of all included patients. Exclusion criteria included patients who had a structural lesion in brain imaging (23 patients), patients with evidence of intracranial infections (7 patients), patients with any other form of secondary headache (36 patients), and patients with altered mental state at any time of hospitalization (58 patients).
According to WHO classification [10], COVID-19 infection was categorized into a mild, moderate or severe infection. The mild state was defined by typical symptoms without evidence of viral pneumonia or hypoxia, while moderate or severe cases were identified if there was any clinical and radiological evidence of pneumonia. In moderate infection, patients had to have SpO2 ≥ 90% on room air while one of the following was required to define the severe cases: respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air.
The treatment protocol followed by the two centers included steroids for moderate and severe cases. IV methylprednisolone 0.5 to 1 mg/kg/day in two divided doses for 3 days was given for moderate cases requiring supplemental oxygen. As for the severe cases, the above regimen of IV methylprednisolone was indicated for 3–7 days [11].
Data collection:The extracted data from hospital medical records included patients’ demographics, comorbidities, manifestations of COVID-19 infection, list of prescribed medications, and laboratory and chest computed tomography (CT) findings.
The evaluated comorbidities included hypertension, diabetes, cardiac diseases, malignancy, hypothyroidism, autoimmune diseases, and hepatic and renal diseases.
List of accompanying symptoms as well as a list of prescribed medications, whether symptomatic treatments or steroids, were extracted. Dehydration was documented if occurred as a sequel to fluid loss in patients presenting with vomiting and\or diarrhea. Definition of dehydration was established if serum osmolarity was greater than 294 mOsm/kg [12].
Initial laboratory results were recorded including neutrophil\lymphocytic ratio (NLR), hemoglobin, platelets, C-reactive protein (CRP), D-dimer, and ferritin. The severity of COVID-19 infection was determined, based on initial CT-chest results along with clinical symptoms.
After recovery and discharge, patients were contacted by phone to revise some of their recorded data including smoking status, body mass index (BMI), comorbidities, and the symptoms of COVID-19 such as headache, fever, and respiratory and gastrointestinal symptoms.
An expert neurologist conducted a semi-structured interview via a phone call for a detailed analysis of headache. Diagnosis of COVID-19 related headache was established according to the ICHD-III criteria of acute headache attributed to systemic viral infection [13]. Patients with COVID-19 related headache were asked about the onset and offset of headache in temporal relation with other COVID-19 symptoms and were also asked to assess their pain intensity using the visual analog scale (VAS) [14]. Headache response to analgesics was also evaluated by the patient’s global impression of improvement. Paracetamol was the painkiller that was prescribed for headache sufferers during the hospital admission. Another neurologist’s role was to assess the presence of pre-existing primary headache disorder according to the ICHD-III criteria [13].
Sampling:Sample size was calculated by using Epi info software, at the confidence level of 95%, α = 0.05, 3% margin of error, and 50% prevalence of COVID-19 related headache. The target population was 2,000 patients who were admitted to Beni-Suef University Hospital and Beni-Suef Insurance Hospital, in the period from April 1 to June 30, 2020, during the ascending arm of the epidemic curve in Egypt. The minimum sample size was calculated to be 696 patients. The patients were selected by systematic random sample technique.
Ethical consideration:Ethical approval was obtained from the Ethical Committee, Faculty of Medicine, Beni-Suef University. The approval number is FMBSUREC/04102020/Hussein_3. Verbal informed consents were taken from the participants. The study was performed according to the principles of the Declaration of Helsinki.
Statistical analysis: IBM SPSS (Statistical Package of Social Science) Version 21 was used to analyze the data, which were tested for normality distribution by using the Kolmogorov-Smirnov test. The characteristics of COVID-19 related headache, clinical and laboratory data of the patients were presented by using frequency and percentage for qualitative data, while median and interquartile range (IQR) were used for not normally distributed quantitative data. The characteristics of the patient with COVID-19 related headache versus the non-headache group and evaluation of treatment response of COVID-19 related headache were assessed by using χ2 tests for categorical variables and Mann-Whitney test for quantitative non-normally distributed variables. A stepwise logistic regression model was done to identify predictors of the occurrence of COVID-19 related headache and predictors of treatment nonresponse of COVID-19 related headache after being adjusted for their potential mutual confounding effect. A P values of less than .05 was considered statistically significant. All tests were two-tailed.
Results
The present study included 782 patients with a confirmed diagnosis of COVID-19 infection. Their ages ranged from 12–85, (median 42 and IQR 31–56). In total, 427 females (54.6%) and 355 males (45.4%) were included. Comorbidities were reported in only 232 (29.7%) patients (Figure 1). Primary headache disorders were reported in only 218 (27.88%) patients; 158 (72.48%) patients had a tension headache, 58 (26.61%) patients had a migraine, while only 2 (0.9%) patients had cluster headache.
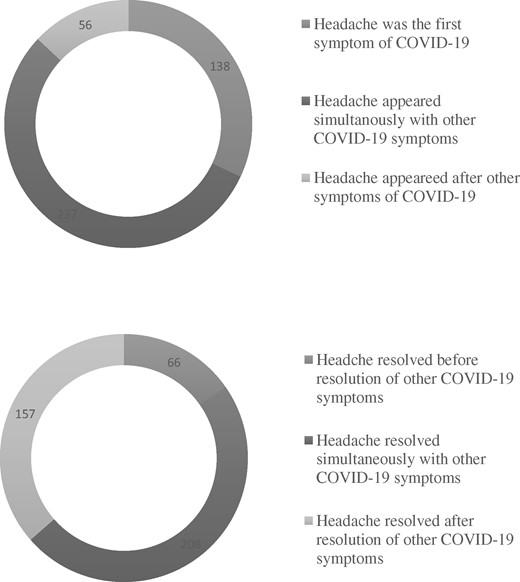
The relative frequency of COVID-19 related headache among our sample was 55.1% (n = 431) with 95% confidence interval (CI) (.516–.586) for the estimated population prevalence. Among the group of headache sufferers, 102 (23.70%) patients had migraine-like phenotype, 224 (52%) patients had tension-like phenotype, and 29 (6.7%) patients had trigeminal autonomic features. However, in 76 (17.6%) patients, we could not characterize the phenotype of their headaches. The median of headache intensity assessed by VAS was 7 with IQR 5–8. Onset and offset of headache in relation to other COVID-19 symptoms were illustrated in Figure 2.
Onset and offset of COVID-19 related headache in relation to other symptoms.
Patients with COVID-19 Related Headache versus the Non-Headache Group
Female gender, primary headache disorders, fever, and dehydration were significantly associated with COVID-19 related headache. All patients with malignancy (n = 7, 100%) experienced headache (P = .016), whereas no significant association was found with other comorbidities such as diabetes, hypertension, cardiac, hypothyroidism, autoimmune, hepatic, or renal diseases.
Regarding laboratory data, patients with COVID-19 related headache had significantly lower levels of hemoglobin and platelets as well as higher levels of NLR and CRP (Table 1).
Comparisons between patients with COVID-19 related headache and non-headache group
| . | COVID-19 patients (n = 782) . | P value . | ||
|---|---|---|---|---|
| Headache group (n = 431) . | Non-headache group (n = 351) . | |||
| Age (Median [IQR]) | 40 (30–56) | 43 (32–56) | .635 | |
| BMI (Median [IQR]) | 27.6 (25.6–31.2) | 28 (25.2–31.2) | .842 | |
| Sex | Males (n = 355) | 177 (41.1%) | 178 (50.7%) | .007 |
| Females (n = 427) | 254 (58.9%) | 173 (49.3%) | ||
| Smoking | Yes (n = 116) | 60 (13.9%) | 56 (15.9%) | .426 |
| No (n = 666) | 371 (86.1%) | 295 (84.1%) | ||
| Primary headache | Yes (n = 218) | 162 (37.6%) | 56 (15.9%) | <.001 |
| No (n = 564) | 269 (62.4%) | 295 (84.1%) | ||
| Fever | Yes (n = 647) | 380 (88.2%) | 267 (76.1%) | <.001 |
| No (n = 135) | 51 (11.8%) | 84 (23.9%) | ||
| Dehydration | Yes (n = 415) | 253 (58.7%) | 162 (46.2%) | <.001 |
| No (n = 367) | 178 (41.3%) | 189 (53.8%) | ||
| COVID-19 severity | Mild (n = 335) | 182 (42.2%) | 153 (53.6%) | .469 |
| Moderate (n = 239) | 127 (29.5%) | 112 (31.9%) | ||
| Severe (n = 208) | 122 (28.3%) | 86 (24.5%) | ||
| Steroids intake | Yes (n = 441) | 237 (55%) | 204 (58.1%) | .380 |
| No (n = 341) | 194 (45%) | 147 (41.9%) | ||
Laboratory data (Median [IQR]) | Hb (gm/dL) | 12 (11–13) | 12 (11–13) | .026 |
| NLR | 3.61 (2.17–5.58) | 3.1 (2.05–4.63) | .002 | |
| Platelets (× 109/L) | 218 (176.5–284.5) | 250.50 (203–313.75) | <.001 | |
| CRP (mg/L) | 33.75 (12–93) | 23.5 (9.25–60.75) | .005 | |
| Ferritin (ng/mL) | 130 (66–300) | 112 (60–256) | .092 | |
| D-dimer (µg/mL) | 0.421 (0.3–0.59) | 0.4 (0.24–0.6) | .160 | |
| . | COVID-19 patients (n = 782) . | P value . | ||
|---|---|---|---|---|
| Headache group (n = 431) . | Non-headache group (n = 351) . | |||
| Age (Median [IQR]) | 40 (30–56) | 43 (32–56) | .635 | |
| BMI (Median [IQR]) | 27.6 (25.6–31.2) | 28 (25.2–31.2) | .842 | |
| Sex | Males (n = 355) | 177 (41.1%) | 178 (50.7%) | .007 |
| Females (n = 427) | 254 (58.9%) | 173 (49.3%) | ||
| Smoking | Yes (n = 116) | 60 (13.9%) | 56 (15.9%) | .426 |
| No (n = 666) | 371 (86.1%) | 295 (84.1%) | ||
| Primary headache | Yes (n = 218) | 162 (37.6%) | 56 (15.9%) | <.001 |
| No (n = 564) | 269 (62.4%) | 295 (84.1%) | ||
| Fever | Yes (n = 647) | 380 (88.2%) | 267 (76.1%) | <.001 |
| No (n = 135) | 51 (11.8%) | 84 (23.9%) | ||
| Dehydration | Yes (n = 415) | 253 (58.7%) | 162 (46.2%) | <.001 |
| No (n = 367) | 178 (41.3%) | 189 (53.8%) | ||
| COVID-19 severity | Mild (n = 335) | 182 (42.2%) | 153 (53.6%) | .469 |
| Moderate (n = 239) | 127 (29.5%) | 112 (31.9%) | ||
| Severe (n = 208) | 122 (28.3%) | 86 (24.5%) | ||
| Steroids intake | Yes (n = 441) | 237 (55%) | 204 (58.1%) | .380 |
| No (n = 341) | 194 (45%) | 147 (41.9%) | ||
Laboratory data (Median [IQR]) | Hb (gm/dL) | 12 (11–13) | 12 (11–13) | .026 |
| NLR | 3.61 (2.17–5.58) | 3.1 (2.05–4.63) | .002 | |
| Platelets (× 109/L) | 218 (176.5–284.5) | 250.50 (203–313.75) | <.001 | |
| CRP (mg/L) | 33.75 (12–93) | 23.5 (9.25–60.75) | .005 | |
| Ferritin (ng/mL) | 130 (66–300) | 112 (60–256) | .092 | |
| D-dimer (µg/mL) | 0.421 (0.3–0.59) | 0.4 (0.24–0.6) | .160 | |
BMI = body mass index; CRP = C-reactive protein; Hb = hemoglobin; IQR = interquartile range; NLR = neutrophil-lymphocyte ratio.
P values < .05 are considered significant.
Comparisons between patients with COVID-19 related headache and non-headache group
| . | COVID-19 patients (n = 782) . | P value . | ||
|---|---|---|---|---|
| Headache group (n = 431) . | Non-headache group (n = 351) . | |||
| Age (Median [IQR]) | 40 (30–56) | 43 (32–56) | .635 | |
| BMI (Median [IQR]) | 27.6 (25.6–31.2) | 28 (25.2–31.2) | .842 | |
| Sex | Males (n = 355) | 177 (41.1%) | 178 (50.7%) | .007 |
| Females (n = 427) | 254 (58.9%) | 173 (49.3%) | ||
| Smoking | Yes (n = 116) | 60 (13.9%) | 56 (15.9%) | .426 |
| No (n = 666) | 371 (86.1%) | 295 (84.1%) | ||
| Primary headache | Yes (n = 218) | 162 (37.6%) | 56 (15.9%) | <.001 |
| No (n = 564) | 269 (62.4%) | 295 (84.1%) | ||
| Fever | Yes (n = 647) | 380 (88.2%) | 267 (76.1%) | <.001 |
| No (n = 135) | 51 (11.8%) | 84 (23.9%) | ||
| Dehydration | Yes (n = 415) | 253 (58.7%) | 162 (46.2%) | <.001 |
| No (n = 367) | 178 (41.3%) | 189 (53.8%) | ||
| COVID-19 severity | Mild (n = 335) | 182 (42.2%) | 153 (53.6%) | .469 |
| Moderate (n = 239) | 127 (29.5%) | 112 (31.9%) | ||
| Severe (n = 208) | 122 (28.3%) | 86 (24.5%) | ||
| Steroids intake | Yes (n = 441) | 237 (55%) | 204 (58.1%) | .380 |
| No (n = 341) | 194 (45%) | 147 (41.9%) | ||
Laboratory data (Median [IQR]) | Hb (gm/dL) | 12 (11–13) | 12 (11–13) | .026 |
| NLR | 3.61 (2.17–5.58) | 3.1 (2.05–4.63) | .002 | |
| Platelets (× 109/L) | 218 (176.5–284.5) | 250.50 (203–313.75) | <.001 | |
| CRP (mg/L) | 33.75 (12–93) | 23.5 (9.25–60.75) | .005 | |
| Ferritin (ng/mL) | 130 (66–300) | 112 (60–256) | .092 | |
| D-dimer (µg/mL) | 0.421 (0.3–0.59) | 0.4 (0.24–0.6) | .160 | |
| . | COVID-19 patients (n = 782) . | P value . | ||
|---|---|---|---|---|
| Headache group (n = 431) . | Non-headache group (n = 351) . | |||
| Age (Median [IQR]) | 40 (30–56) | 43 (32–56) | .635 | |
| BMI (Median [IQR]) | 27.6 (25.6–31.2) | 28 (25.2–31.2) | .842 | |
| Sex | Males (n = 355) | 177 (41.1%) | 178 (50.7%) | .007 |
| Females (n = 427) | 254 (58.9%) | 173 (49.3%) | ||
| Smoking | Yes (n = 116) | 60 (13.9%) | 56 (15.9%) | .426 |
| No (n = 666) | 371 (86.1%) | 295 (84.1%) | ||
| Primary headache | Yes (n = 218) | 162 (37.6%) | 56 (15.9%) | <.001 |
| No (n = 564) | 269 (62.4%) | 295 (84.1%) | ||
| Fever | Yes (n = 647) | 380 (88.2%) | 267 (76.1%) | <.001 |
| No (n = 135) | 51 (11.8%) | 84 (23.9%) | ||
| Dehydration | Yes (n = 415) | 253 (58.7%) | 162 (46.2%) | <.001 |
| No (n = 367) | 178 (41.3%) | 189 (53.8%) | ||
| COVID-19 severity | Mild (n = 335) | 182 (42.2%) | 153 (53.6%) | .469 |
| Moderate (n = 239) | 127 (29.5%) | 112 (31.9%) | ||
| Severe (n = 208) | 122 (28.3%) | 86 (24.5%) | ||
| Steroids intake | Yes (n = 441) | 237 (55%) | 204 (58.1%) | .380 |
| No (n = 341) | 194 (45%) | 147 (41.9%) | ||
Laboratory data (Median [IQR]) | Hb (gm/dL) | 12 (11–13) | 12 (11–13) | .026 |
| NLR | 3.61 (2.17–5.58) | 3.1 (2.05–4.63) | .002 | |
| Platelets (× 109/L) | 218 (176.5–284.5) | 250.50 (203–313.75) | <.001 | |
| CRP (mg/L) | 33.75 (12–93) | 23.5 (9.25–60.75) | .005 | |
| Ferritin (ng/mL) | 130 (66–300) | 112 (60–256) | .092 | |
| D-dimer (µg/mL) | 0.421 (0.3–0.59) | 0.4 (0.24–0.6) | .160 | |
BMI = body mass index; CRP = C-reactive protein; Hb = hemoglobin; IQR = interquartile range; NLR = neutrophil-lymphocyte ratio.
P values < .05 are considered significant.
Evaluation of Treatment Response of COVID-19 Related Headache
Among the headache sufferers, 344 patients (79.8%) reported good responses to analgesics, while only 87 patients (20.2%) reported that the analgesics were ineffective. By evaluating headache response to analgesics, the non-responders group had significantly higher CRP and ferritin levels as well as lower hemoglobin levels. Moreover, old age, comorbidities, primary headache, and severe COVID-19 infection were associated with poor response, whereas steroids intake was associated with good response (Table 2).
Comparisons between responders and non-responders groups to analgesics
| . | Response of COVID-19 related headache to analgesics (n = 431) . | P value . | ||
|---|---|---|---|---|
| Responders group (n = 344) . | Non-responders group(n = 87) . | |||
| Age (Median [IQR]) | 39 (30–55) | 46 (33–62) | .012 | |
| BMI (Median [IQR]) | 27.54 (25.3 – 31.1) | 28.41 (26.5– 33.3) | .075 | |
| Sex | Males (n = 177) | 143 (41.6%) | 34 (39.1%) | .673 |
| Females (n = 254) | 201 (58.4%) | 53 (60.9%) | ||
| Smoking | Yes (n = 60) | 49 (14.2%) | 11 (12.6%) | .7 |
| No (n = 371) | 295 (85.8%) | 76 (87.4%) | ||
| Primary headache | Yes (n = 162) | 116 (33.7%) | 46 (52.9%) | .001 |
| No (n = 269) | 228 (66.3%) | 41 (47.1%) | ||
| Fever | Yes (n = 380) | 307 (89.2%) | 73 (83.9%) | .169 |
| No (n = 51) | 37 (10.8%) | 14 (16.1%) | ||
| Dehydration | Yes (n = 253) | 200 (58.1) | 53 (60.9%) | .638 |
| No (n = 178) | 144 (41.9) | 34 (39.1%) | ||
| COVID-19 severity | Mild (n = 182) | 156 (45.3%) | 26 (29.9%) | .014 |
| Moderate (n = 127) | 100 (29.1%) | 27 (31%) | ||
| Severe (n = 122) | 88 (25.6%) | 34 (39.1%) | ||
| Steroids intake | Yes (n = 237) | 199 (57.8%) | 38 (43.7%) | .018 |
| No (n = 194) | 145 (42.2%) | 49 (56.3%) | ||
| Laboratory data [Median (IQR)] | Hb (gm\dL) | 12 (11 – 13) | 11.7 (10.65–12.9) | .022 |
| NLR | 3.56 (2.17–5.42) | 4.24 (2.35–6.25) | .187 | |
| Platelets (× 109/L) | 220 (180 – 288) | 203.5 (162.75–259.2) | .100 | |
| CRP (mg/L) | 24 (12 – 66) | 48 (24–96) | <.001 | |
| Ferritin (ng/mL) | 120 (64–292) | 207 (96–380) | .038 | |
| D-dimer (µg/mL) | 0.408 (0.3 – 0.59) | 0.5 (0.4–0.62) | .105 | |
| . | Response of COVID-19 related headache to analgesics (n = 431) . | P value . | ||
|---|---|---|---|---|
| Responders group (n = 344) . | Non-responders group(n = 87) . | |||
| Age (Median [IQR]) | 39 (30–55) | 46 (33–62) | .012 | |
| BMI (Median [IQR]) | 27.54 (25.3 – 31.1) | 28.41 (26.5– 33.3) | .075 | |
| Sex | Males (n = 177) | 143 (41.6%) | 34 (39.1%) | .673 |
| Females (n = 254) | 201 (58.4%) | 53 (60.9%) | ||
| Smoking | Yes (n = 60) | 49 (14.2%) | 11 (12.6%) | .7 |
| No (n = 371) | 295 (85.8%) | 76 (87.4%) | ||
| Primary headache | Yes (n = 162) | 116 (33.7%) | 46 (52.9%) | .001 |
| No (n = 269) | 228 (66.3%) | 41 (47.1%) | ||
| Fever | Yes (n = 380) | 307 (89.2%) | 73 (83.9%) | .169 |
| No (n = 51) | 37 (10.8%) | 14 (16.1%) | ||
| Dehydration | Yes (n = 253) | 200 (58.1) | 53 (60.9%) | .638 |
| No (n = 178) | 144 (41.9) | 34 (39.1%) | ||
| COVID-19 severity | Mild (n = 182) | 156 (45.3%) | 26 (29.9%) | .014 |
| Moderate (n = 127) | 100 (29.1%) | 27 (31%) | ||
| Severe (n = 122) | 88 (25.6%) | 34 (39.1%) | ||
| Steroids intake | Yes (n = 237) | 199 (57.8%) | 38 (43.7%) | .018 |
| No (n = 194) | 145 (42.2%) | 49 (56.3%) | ||
| Laboratory data [Median (IQR)] | Hb (gm\dL) | 12 (11 – 13) | 11.7 (10.65–12.9) | .022 |
| NLR | 3.56 (2.17–5.42) | 4.24 (2.35–6.25) | .187 | |
| Platelets (× 109/L) | 220 (180 – 288) | 203.5 (162.75–259.2) | .100 | |
| CRP (mg/L) | 24 (12 – 66) | 48 (24–96) | <.001 | |
| Ferritin (ng/mL) | 120 (64–292) | 207 (96–380) | .038 | |
| D-dimer (µg/mL) | 0.408 (0.3 – 0.59) | 0.5 (0.4–0.62) | .105 | |
BMI = body mass index; CRP = C-reactive protein; Hb = hemoglobin; IQR = interquartile range; NLR = neutrophil-lymphocyte ratio.
P values < .05 are considered significant.
Comparisons between responders and non-responders groups to analgesics
| . | Response of COVID-19 related headache to analgesics (n = 431) . | P value . | ||
|---|---|---|---|---|
| Responders group (n = 344) . | Non-responders group(n = 87) . | |||
| Age (Median [IQR]) | 39 (30–55) | 46 (33–62) | .012 | |
| BMI (Median [IQR]) | 27.54 (25.3 – 31.1) | 28.41 (26.5– 33.3) | .075 | |
| Sex | Males (n = 177) | 143 (41.6%) | 34 (39.1%) | .673 |
| Females (n = 254) | 201 (58.4%) | 53 (60.9%) | ||
| Smoking | Yes (n = 60) | 49 (14.2%) | 11 (12.6%) | .7 |
| No (n = 371) | 295 (85.8%) | 76 (87.4%) | ||
| Primary headache | Yes (n = 162) | 116 (33.7%) | 46 (52.9%) | .001 |
| No (n = 269) | 228 (66.3%) | 41 (47.1%) | ||
| Fever | Yes (n = 380) | 307 (89.2%) | 73 (83.9%) | .169 |
| No (n = 51) | 37 (10.8%) | 14 (16.1%) | ||
| Dehydration | Yes (n = 253) | 200 (58.1) | 53 (60.9%) | .638 |
| No (n = 178) | 144 (41.9) | 34 (39.1%) | ||
| COVID-19 severity | Mild (n = 182) | 156 (45.3%) | 26 (29.9%) | .014 |
| Moderate (n = 127) | 100 (29.1%) | 27 (31%) | ||
| Severe (n = 122) | 88 (25.6%) | 34 (39.1%) | ||
| Steroids intake | Yes (n = 237) | 199 (57.8%) | 38 (43.7%) | .018 |
| No (n = 194) | 145 (42.2%) | 49 (56.3%) | ||
| Laboratory data [Median (IQR)] | Hb (gm\dL) | 12 (11 – 13) | 11.7 (10.65–12.9) | .022 |
| NLR | 3.56 (2.17–5.42) | 4.24 (2.35–6.25) | .187 | |
| Platelets (× 109/L) | 220 (180 – 288) | 203.5 (162.75–259.2) | .100 | |
| CRP (mg/L) | 24 (12 – 66) | 48 (24–96) | <.001 | |
| Ferritin (ng/mL) | 120 (64–292) | 207 (96–380) | .038 | |
| D-dimer (µg/mL) | 0.408 (0.3 – 0.59) | 0.5 (0.4–0.62) | .105 | |
| . | Response of COVID-19 related headache to analgesics (n = 431) . | P value . | ||
|---|---|---|---|---|
| Responders group (n = 344) . | Non-responders group(n = 87) . | |||
| Age (Median [IQR]) | 39 (30–55) | 46 (33–62) | .012 | |
| BMI (Median [IQR]) | 27.54 (25.3 – 31.1) | 28.41 (26.5– 33.3) | .075 | |
| Sex | Males (n = 177) | 143 (41.6%) | 34 (39.1%) | .673 |
| Females (n = 254) | 201 (58.4%) | 53 (60.9%) | ||
| Smoking | Yes (n = 60) | 49 (14.2%) | 11 (12.6%) | .7 |
| No (n = 371) | 295 (85.8%) | 76 (87.4%) | ||
| Primary headache | Yes (n = 162) | 116 (33.7%) | 46 (52.9%) | .001 |
| No (n = 269) | 228 (66.3%) | 41 (47.1%) | ||
| Fever | Yes (n = 380) | 307 (89.2%) | 73 (83.9%) | .169 |
| No (n = 51) | 37 (10.8%) | 14 (16.1%) | ||
| Dehydration | Yes (n = 253) | 200 (58.1) | 53 (60.9%) | .638 |
| No (n = 178) | 144 (41.9) | 34 (39.1%) | ||
| COVID-19 severity | Mild (n = 182) | 156 (45.3%) | 26 (29.9%) | .014 |
| Moderate (n = 127) | 100 (29.1%) | 27 (31%) | ||
| Severe (n = 122) | 88 (25.6%) | 34 (39.1%) | ||
| Steroids intake | Yes (n = 237) | 199 (57.8%) | 38 (43.7%) | .018 |
| No (n = 194) | 145 (42.2%) | 49 (56.3%) | ||
| Laboratory data [Median (IQR)] | Hb (gm\dL) | 12 (11 – 13) | 11.7 (10.65–12.9) | .022 |
| NLR | 3.56 (2.17–5.42) | 4.24 (2.35–6.25) | .187 | |
| Platelets (× 109/L) | 220 (180 – 288) | 203.5 (162.75–259.2) | .100 | |
| CRP (mg/L) | 24 (12 – 66) | 48 (24–96) | <.001 | |
| Ferritin (ng/mL) | 120 (64–292) | 207 (96–380) | .038 | |
| D-dimer (µg/mL) | 0.408 (0.3 – 0.59) | 0.5 (0.4–0.62) | .105 | |
BMI = body mass index; CRP = C-reactive protein; Hb = hemoglobin; IQR = interquartile range; NLR = neutrophil-lymphocyte ratio.
P values < .05 are considered significant.
Regarding comorbidities, 68.18% (n = 45) of diabetic patients were responders to analgesics versus 81.92% (n = 299) in non-diabetic patients (P = .011). Also, 67.57% (n = 50) of hypertensive patients were responders to analgesics versus 82.35% (n = 294) in non-hypertensive patients (P = 0.004). On the other hand, other comorbidities such as cardiac diseases, hypothyroidism, malignancy, autoimmune, and hepatic or renal diseases did not show a significant impact on the response of headache to analgesics.
Risk Factors of Occurrence COVID-19 Related Headache by Multivariate Analysis
Stepwise logistic regression was done by using the following variables: age, sex, smoking, BMI, malignancy, fever, dehydration, primary headache, steroids intake, hemoglobin, NLR, platelets, CRP, ferritin, D-dimer, the severity of COVID-19 infection (moderate vs mild and severe vs mild).
Only primary headache, sex, fever, dehydration, NLR, and platelets were retained as independent predictors of occurrence COVID-19 related headache. It was found that primary headache disorders, being female, fever, and dehydration, increased odds of headache occurrence by 2.637, 2.55, 2.246, and 1.918 times, respectively. Also, each unit increase in NLR increased odds of headache occurrence by 1.344, while each unit decrease in platelets increased odds of occurrence of headache by 0.995 (Table 3).
Stepwise logistic regression to detect predictors of occurrence of COVID-19 related headache
| . | B . | SE . | P . | OR . | 95.0% CI . | |
|---|---|---|---|---|---|---|
| Lower . | Upper . | |||||
| Sex | −0.937 | 0.307 | .002 | 2.55 | 1.39 | 4.658 |
| Fever | 0.809 | 0.365 | .027 | 2.246 | 1.099 | 4.590 |
| Dehydration | 0.651 | 0.306 | .033 | 1.918 | 1.054 | 3.492 |
| Primary headache | 0.970 | 0.319 | .002 | 2.637 | 1.410 | 4.932 |
| NLR | 0.296 | 0.071 | .000 | 1.344 | 1.169 | 1.546 |
| Platelets | −0.005 | 0.002 | .009 | 0.995 | 0.992 | 0.999 |
| Constant | −0.785 | 0.640 | .220 | 0.456 | ||
| . | B . | SE . | P . | OR . | 95.0% CI . | |
|---|---|---|---|---|---|---|
| Lower . | Upper . | |||||
| Sex | −0.937 | 0.307 | .002 | 2.55 | 1.39 | 4.658 |
| Fever | 0.809 | 0.365 | .027 | 2.246 | 1.099 | 4.590 |
| Dehydration | 0.651 | 0.306 | .033 | 1.918 | 1.054 | 3.492 |
| Primary headache | 0.970 | 0.319 | .002 | 2.637 | 1.410 | 4.932 |
| NLR | 0.296 | 0.071 | .000 | 1.344 | 1.169 | 1.546 |
| Platelets | −0.005 | 0.002 | .009 | 0.995 | 0.992 | 0.999 |
| Constant | −0.785 | 0.640 | .220 | 0.456 | ||
R2 0.327, dependent variable: occurrence of COVID-19 related headache.
CI = confidence interval; OR = odds ratio.
P values < .05 are considered significant.
Stepwise logistic regression to detect predictors of occurrence of COVID-19 related headache
| . | B . | SE . | P . | OR . | 95.0% CI . | |
|---|---|---|---|---|---|---|
| Lower . | Upper . | |||||
| Sex | −0.937 | 0.307 | .002 | 2.55 | 1.39 | 4.658 |
| Fever | 0.809 | 0.365 | .027 | 2.246 | 1.099 | 4.590 |
| Dehydration | 0.651 | 0.306 | .033 | 1.918 | 1.054 | 3.492 |
| Primary headache | 0.970 | 0.319 | .002 | 2.637 | 1.410 | 4.932 |
| NLR | 0.296 | 0.071 | .000 | 1.344 | 1.169 | 1.546 |
| Platelets | −0.005 | 0.002 | .009 | 0.995 | 0.992 | 0.999 |
| Constant | −0.785 | 0.640 | .220 | 0.456 | ||
| . | B . | SE . | P . | OR . | 95.0% CI . | |
|---|---|---|---|---|---|---|
| Lower . | Upper . | |||||
| Sex | −0.937 | 0.307 | .002 | 2.55 | 1.39 | 4.658 |
| Fever | 0.809 | 0.365 | .027 | 2.246 | 1.099 | 4.590 |
| Dehydration | 0.651 | 0.306 | .033 | 1.918 | 1.054 | 3.492 |
| Primary headache | 0.970 | 0.319 | .002 | 2.637 | 1.410 | 4.932 |
| NLR | 0.296 | 0.071 | .000 | 1.344 | 1.169 | 1.546 |
| Platelets | −0.005 | 0.002 | .009 | 0.995 | 0.992 | 0.999 |
| Constant | −0.785 | 0.640 | .220 | 0.456 | ||
R2 0.327, dependent variable: occurrence of COVID-19 related headache.
CI = confidence interval; OR = odds ratio.
P values < .05 are considered significant.
Risk Factors of Treatment Non-Response of COVID-19 Related Headache by Multivariate Analysis
Stepwise logistic regression was done by using the following variables: age, sex, smoking, BMI, diabetes, hypertension, fever, dehydration, primary headache, steroids intake, hemoglobin, NLR, platelets, CRP, ferritin, and severity of COVID-19 infection (moderate vs mild and severe vs mild).
Only primary headache, steroids intake, moderate, and severe COVID-19 infection were retained as independent predictors of non-response of COVID-19 related headache to analgesics. It was found that primary headache disorders, moderate, and severe COVID-19 infection increased the odds of non-response to analgesics by 2.684, 5.114, and 8.062 times, respectively. However, steroids intake was associated with decreased odds of non-response to analgesics by 0.339 times (Table 4).
Stepwise logistic regression to detect predictors of non-response of COVID-19 related headache to analgesics
| . | B . | SE . | P . | OR . | 95.0% CI . | ||
|---|---|---|---|---|---|---|---|
| Lower . | Upper . | ||||||
| Primary headache | 0.987 | 0.400 | 0.014 | 2.684 | 1.226 | 5.875 | |
| Steroids intake | −1.082 | 0.433 | 0.012 | 0.339 | 0.145 | 0.791 | |
| COVID-19 Severity of | Moderate | 1.632 | 0.581 | 0.005 | 5.114 | 1.636 | 15.984 |
| Severe | 2.087 | 0.587 | <0.001 | 8.062 | 2.550 | 25.490 | |
| Constant | −2.780 | 0.550 | <0.001 | 0.062 | |||
| . | B . | SE . | P . | OR . | 95.0% CI . | ||
|---|---|---|---|---|---|---|---|
| Lower . | Upper . | ||||||
| Primary headache | 0.987 | 0.400 | 0.014 | 2.684 | 1.226 | 5.875 | |
| Steroids intake | −1.082 | 0.433 | 0.012 | 0.339 | 0.145 | 0.791 | |
| COVID-19 Severity of | Moderate | 1.632 | 0.581 | 0.005 | 5.114 | 1.636 | 15.984 |
| Severe | 2.087 | 0.587 | <0.001 | 8.062 | 2.550 | 25.490 | |
| Constant | −2.780 | 0.550 | <0.001 | 0.062 | |||
R2 0.206, dependent variable: non-response of COVID-19 related headache to analgesics.
CI = confidence interval; OR = odds ratio.
P values < .05 are considered significant.
Stepwise logistic regression to detect predictors of non-response of COVID-19 related headache to analgesics
| . | B . | SE . | P . | OR . | 95.0% CI . | ||
|---|---|---|---|---|---|---|---|
| Lower . | Upper . | ||||||
| Primary headache | 0.987 | 0.400 | 0.014 | 2.684 | 1.226 | 5.875 | |
| Steroids intake | −1.082 | 0.433 | 0.012 | 0.339 | 0.145 | 0.791 | |
| COVID-19 Severity of | Moderate | 1.632 | 0.581 | 0.005 | 5.114 | 1.636 | 15.984 |
| Severe | 2.087 | 0.587 | <0.001 | 8.062 | 2.550 | 25.490 | |
| Constant | −2.780 | 0.550 | <0.001 | 0.062 | |||
| . | B . | SE . | P . | OR . | 95.0% CI . | ||
|---|---|---|---|---|---|---|---|
| Lower . | Upper . | ||||||
| Primary headache | 0.987 | 0.400 | 0.014 | 2.684 | 1.226 | 5.875 | |
| Steroids intake | −1.082 | 0.433 | 0.012 | 0.339 | 0.145 | 0.791 | |
| COVID-19 Severity of | Moderate | 1.632 | 0.581 | 0.005 | 5.114 | 1.636 | 15.984 |
| Severe | 2.087 | 0.587 | <0.001 | 8.062 | 2.550 | 25.490 | |
| Constant | −2.780 | 0.550 | <0.001 | 0.062 | |||
R2 0.206, dependent variable: non-response of COVID-19 related headache to analgesics.
CI = confidence interval; OR = odds ratio.
P values < .05 are considered significant.
Discussion
This is the first two-center, hospital-based study in the Middle East to clarify the effect of demographic, clinical, and laboratory characteristics on the occurrence of COVID-19 related headache.
The prevalence of COVID-19 related headache varied among different populations. While the Chinese studies found a low prevalence (12%) [5], a high prevalence (70%) was reported by the European studies [6]. In the present study, the relative frequency of COVID-19 related headache in our sample of the Egyptian population was 55.1%, which is closer to the European population. This discrepancy may be due to genetic differences in expression of the angiotensin-converting enzyme 2 (ACE2) receptors [15, 16].
In the current study, the presence of primary headache was associated with increased odds of headache occurrence as well as response to analgesics by 2.637 and 2.684 times, respectively. It is well known that patients with primary headache disorders are more prone to developing secondary headaches [17, 18]. Primary headache disorders are considered demodulatory or dysfunctional painful syndromes in which there is an altered central nervous system functioning [19, 20]. Neurophysiologic studies showed that even in the interictal period, migraine patients have hypersensitivity to painful stimuli, which is characterized by increased amplitude and decreased habituation of event-related evoked potentials [21, 22].
In our study, fever was found to increase the odds of headache occurrence by 2.246 times in patients with COVID-19. This might be attributed to the increases in the prostaglandins E2 level in patients with fever, which has vasoactive properties and could be indirectly implicated in the vascular component of headache. In feverish patients, there was also a reported increase in the release of interleukins, which activate the serotonergic brainstem nuclei implicated in headache induction [23, 24].
Furthermore, we found that dehydration increased the odds of headache occurrence by 1.918 times. Such association might be attributed to decreased plasma volume, increased plasma osmolarity, and increased renin activity in dehydrated patients which all consequently cause intracranial dehydration and low intracranial pressure headache [25, 26].
Medical comorbidities were reported in the literature to be not only risk factors for headache but also predictors of its severity [27]. In general, headache disorders have been linked to various medical disorders, such as hypertension, diabetes, and hypothyroidism [28–31]. In our study, we found that diabetes and hypertension were significantly associated with poor response of headaches to analgesics. We also found that all patients suffering from malignancy experienced COVID-19 related headache. This was probably attributed to the direct immune-mediated effect of malignancy on endothelial cells. Cerebral microscopic findings in patients with malignancy showed that the small cerebral arteries were surrounded by inflammatory infiltrates [32, 33].
It has been speculated that COVID-19 is accompanied by the release of pro-inflammatory cytokines including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [34], which is called the cytokine storm. Such pro-inflammatory cytokines are involved in multiple pathological pain states [35]. The current study revealed a significantly better response of COVID-19 related headache to analgesics in the group of patients who received steroids in comparison to those who did not receive. Also, the odds of non-response to analgesics was lowered by 0.339 times by using steroids. So, we can conclude that steroid might have a role in the improvement of COVID-19 related headache. Additionally, higher CRP and ferritin levels were associated with poor response of headache to analgesics. Taken together, all these factors may indicate a possible inflammatory/immunological mechanism of COVID-19 related headache.
High NLR is considered one of the most evident factors indicating the presence of severe COVID-19 infection. It reflects the degree of imbalance between inflammatory and immune responses. One of the most convincing explanations is based on the association between lymphopenia and neutrophilia and systemic inflammation. Lymphocytes are key regulators of the inflammatory response, and their reduction is associated with the non-resolution of inflammation [36, 37]. In the present study, we found that each unit increase in NLR increased the odds of headache occurrence by 1.344 times.
The current study revealed that lower platelet count was associated with a high frequency of COVID-19 related headache. Headache was frequently reported as one of the neurological manifestations of thrombocytopenia. The possible mechanisms include increased plasma levels of serotonin and hypersensitivity of serotonin receptors, in addition to increased calcitonin gene-related peptide and platelet adenosine diphosphate levels [38–41].
A recent meta-analysis showed that the hemoglobin level was significantly reduced in patients with severe COVID-19 in comparison to those with milder form. Lower hemoglobin level was also found to be a predictive factor of worse clinical outcome of COVID-19 [42]. In the present study, lower hemoglobin level was associated with a high frequency of COVID-19 related headache and poor response of headache to analgesics.
In general, Keith et al. demonstrated that obesity is a risk factor for headaches. Obesity is considered a pro-inflammatory and pro-thrombotic state as well [43, 44]. Adipocytes secrete a wide range of inflammatory mediators, including TNF-B and IL-6 [45]. However, we did not find any effect of BMI on headache occurrence in COVID-19 patients.
Cigarette smoking has been shown to induce several pathophysiological changes, which may trigger headache occurrence. Nicotine is known to have powerful vasoactive properties [46], and carbon monoxide, a byproduct of cigarette smoke, can induce vascular changes due to anoxia and subsequently trigger headache [47]. Nevertheless, it was found that smoking did not affect headache occurrence in COVID-19 patients.
The strength of our study is that it is the first study to clarify the frequency of COVID-19 related headache among the Egyptian population and the impact of clinical and laboratory findings of COVID-19 on headache occurrence and its response to analgesics. Our study has some limitations; the first is the retrospective collection of data from COVID-19 patients. Therefore, information about headache in patients who died was not available. Second, we did not evaluate whether the prognosis of COVID-19 differed in patients with headache compared to those without headache. Thirdly, no data were obtained from the included patients regarding the presence of chronic pain disorders, which may be potential risk factors for COVID-19 related headache.
Clinical Significance
This work sheds light on the relative frequency of COVID-19 related headache among the Egyptian population. It also highlighted the clinical and laboratory predictors of COVID-19 related headache occurrence and its response to analgesics.
Conclusion
The relative frequency of COVID-19 related headache in our sample of the Egyptian population was 55.1%. Female gender, primary headache, fever, dehydration as well as some laboratory findings as higher levels of NLR and decreased platelet count could be predictors of COVID-19 related headache. Primary headache, moderate and severe COVID-19 could be predictors of poor response to analgesics, while steroid intake increased the likelihood of response to analgesics.
Competing Interests
Authors have no competing interest, and the work was not supported by any organization.
Availability of Data and Materials
Authors report that the data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ Contribution
M.H. participated in study design, collection and interpretation of data and helped to draft manuscript. W.F. participated in study design, collection and interpretation of data and helped to draft manuscript. R.E. participated in collection and interpretation of data and helped to draft manuscript. H.A. participated in collection of data and helped to draft manuscript. A.Y. participated in study design, collection and interpretation of data and helped to draft manuscript. M.S. participated in study design, collection and interpretation of data and helped to draft manuscript. C.R. participated in study design, sequence alignment and helped to draft manuscript. A.D. participated in study design, collection and interpretation of data and helped to draft manuscript. H.R. participated in study design, analysis and interpretation of data and helped to draft manuscript. N.L. participated in study design and revised the manuscript. R.M. participated in study design, collection and interpretation of data and helped to draft manuscript. All authors read and approved the final manuscript.
Funding sources: Authors did not receive any funding for this work.
Conflicts of interest: There are no conflicts of interest to report.
Acknowledgments
Not applicable
References
World Health Organization. Clinical management of COVID-19: interim guidance, 27 May 2020. World Health Organization;
Health UDo, Services H. Nicotine: Sites and Mechanisms of Action. The Health Consequences of Smoking Nicotine Addiction a Report of the Surgeon General (DHHS Publication No. CDC 88-8406) Washington, DC: US Government Printing Office;