-
PDF
- Split View
-
Views
-
Cite
Cite
Allan Wailoo, Mónica Hernández Alava, Ian C. Scott, Fowzia Ibrahim, David L. Scott, Cost-effectiveness of treatment strategies using combination disease-modifying anti-rheumatic drugs and glucocorticoids in early rheumatoid arthritis, Rheumatology, Volume 53, Issue 10, October 2014, Pages 1773–1777, https://doi.org/10.1093/rheumatology/keu039
Close - Share Icon Share
Abstract
Objective. The aim of this study was to estimate the cost-effectiveness of combination DMARDs with short-term glucocorticoids in early active RA using data from the 2-year Combination of Anti-Rheumatic Drugs in Early RA (CARDERA) trial.
Methods. CARDERA enrolled 467 patients with active RA of <24-months duration. All patients received MTX; half received step-down prednisolone and half ciclosporin in a placebo-controlled factorial design. Differences in mean costs and quality-adjusted life-years (QALYs) over 24-months follow-up were estimated using patient-level data from a UK health service perspective and 2011–12 costs.
Results. Two-year costs for each treatment strategy showed primary care costs were negligible across all groups. Drug costs were lowest with MTX/ciclosporin and triple therapy. Hospital costs were lowest with MTX/prednisolone and triple therapy. Triple therapy was least costly and most effective; it dominated all other strategies. At positive values for a QALY in the typical UK range (£20 000–30 000) the probability that triple therapy was the most cost-effective strategy was 0.9. Results were robust to methods used to impute missing data.
Conclusion. Intensive treatment of early RA with triple therapy (two DMARDs and short-term glucocorticoids) is both clinically effective and cost effective.
Introduction
There is extensive evidence that treatment strategies using combination DMARDs are effective in early active RA [1–3]. Glucocorticoids enhance their benefits [4]. British, French, European and North American guidelines advocate the use of initial combination DMARDs in most patients with early RA [5–8]. In England and Wales, the National Institute for Health and Care Excellence (NICE) recommends offering patients with early active RA DMARD combinations including MTX alongside short-term glucocorticoids [6].
The economic evidence underpinning the NICE guidance came from a decision model bringing together data from a range of clinical trials grouped by their strategies for combining DMARDs [9]. The most cost-effective combination strategy was considered downward titration of intensive initial triple therapy. Such economic modelling helps decision makers by synthesising complex evidence from multiple clinical trials and other data sources in a coherent analytic framework. However, this comes at the expense of loss of some of the detail in the data from the individual contributing studies. For example, observed resource use data within the trials was not used to cost out broad treatment strategies in the decision model. Economic analysis that makes greater use of this individual patient-level data can both confirm and establish cost-effective DMARD combinations. This has been undertaken alongside three clinical trials: the Tight Control of Rheumatic Arthritis (TICORA), Combinatietherapie Bij Reumatoïde Artritis (COBRA) and BeST studies [10–12]. Each show combination DMARDs are likely to be cost effective, but only TICORA is based on resource use in the UK National Health Service (NHS).
We have undertaken a health economic assessment of combination DMARDs with short-term glucocorticoids in patients with early active RA previously enrolled to the Combination of Anti-Rheumatic Drugs in Early RA (CARDERA) trial. This 2-year double-blind, factorial-designed, placebo-controlled randomized trial compared the benefits of adding ciclosporin, high-dose step-down prednisolone or both to MTX monotherapy [4]. Detailed data was collected on resource use and health-related quality of life (HRQoL), which have not previously been analysed. Our aim was to establish whether combination DMARDs using MTX, ciclosporin and glucocorticoids represent a cost-effective treatment strategy in early active RA patients.
Patients and methods
Patients, interventions and clinical assessments
The methods and clinical results from the CARDERA trial have previously been published [4]. The trial enrolled 467 adult patients with active RA of <24-months duration. All patients received MTX (target dose 15 mg/week) with folic acid. It had a factorial design with patients randomized to receive (i) step-down prednisolone started with MTX (60 mg/day initially, reduced to 7.5 mg at 6 weeks, 7.5 mg/day from 6 to 28 weeks, stopped by 34 weeks) or matching placebo, and (ii) ciclosporin started 3 months after MTX (initial dose 100 mg/day, increased gradually to target dose of 3 mg/kg/day) or matching placebo. Patients were followed up for 2 years. The main outcomes were erosive progression, changes in Larsen X-ray scores, changes in disease activity and changes in the HAQ. The CARDERA trial and accompanying economic evaluation reported here were approved by the South Thames Multicentre Research Ethics Committee [REC reference MREC(1)99/04] and local research ethics committees at each centre. All patients gave written informed consent.
Economic analysis
We estimated differences in costs and quality-adjusted life-years (QALYs) using patient-level data collected as part of the CARDERA trial from a health care perspective. We focussed on HRQoL as the key component for the economic analysis. QALYs were used to reflect benefits to patients in terms of both mortality and morbidity and to allow decision makers to compare interventions competing for scarce health care resources across a wide range of disease areas using a common currency.
Resource use
Detailed resource use data relating to RA were collected in 6-month blocks at months 6, 12, 18 and 24. This covered the following items based on a patient survey that was completed by the specialist nurse alongside the patient: prescription drugs; outpatient, inpatient and day-case hospitalizations (both in rheumatology and other hospital departments); tests; imaging and surgical procedures, including orthopaedic surgery; and primary and community care visits according to the type of health care professional. Study drug dose and frequency were monitored throughout. Details of resource use by category and the proportion of patients using these resources are provided in supplementary Table S1, available at Rheumatology Online.
Unit costs
Resource use categories were costed using figures for 2011–12. Prescription drugs were based on the British National Formulary [13]. Hospital-based services were estimated using NHS reference costs [14]. Orthopaedic surgery was based on reference costs per day for hip, knee, foot, shoulder and arm procedures and weighted according to the total number of finished consultant episodes. Primary and community care services were based on Curtis [15].
Health outcomes
The EQ-5D is a generic, five-dimension measure of HRQoL, widely used and accepted as valid in RA, which provides a summary score ranging from −0.6 to 1 based on UK public preferences for the 243 health states it can describe. EQ-5D was administered to patients at baseline, 6, 12, 18 and 24 months. Total QALYs over 2 years were calculated as the area under the curve assuming linear interpolation between the five time points.
Statistical analysis
Costs and QALYs were calculated over a 2-year period with a discount rate of 3.5% applied to those occurring in year 2. Mean costs and QALYs and their 95% CIs were calculated for each of the four treatment arms. The incremental cost-effectiveness ratio was calculated where appropriate, based on standard decision rules.
Two hundred and forty-two (54%) patients had complete data for all items of cost and HRQoL at all follow-up times, as well as baseline data. To avoid a loss in efficiency, missing values were imputed using multiple imputation by chained equations, but baseline covariates were imputed using mean imputation to preserve their independence of treatment [16]. No significant differences in baseline characteristics were observed between complete and non-complete cases. One hundred imputed datasets were simulated using a set of imputation models including the variables total QALYs, total costs, age, sex, ethnicity, disease duration, dummies for the trial arm and region and baseline 28-joint DAS (DAS28), Larsen and HAQ. A shifted lognormal transformation of total costs [ln(C − 1297.94)] was used in the imputation models to remove the skewness that typically characterises cost data and later reversed after imputation. Total QALYs were imputed using predictive mean matching with one nearest neighbour, as regression models were found to impute values outside the feasible range. Covariates were used in the imputation model even for carrying out unadjusted analyses. The analyses were performed in each imputed dataset and the estimated parameters of interest combined using Rubin’s rule [17]. All analyses were carried out in Stata version 12 (StataCorp, College Station, TX, USA).
Bootstrap methods were applied to the imputed datasets in order to calculate cost-effectiveness acceptability curves for threshold values between £0 and £100 000 per QALY. NICE operates a reported threshold of £20 000–£30 000 per QALY for most therapies. The robustness of results according to the method used for imputation, the number of imputation datasets and the number of bootstrap replications were examined.
Based on bootstrap methods, 95% CIs were calculated to overcome issues with the non-normal distribution of costs, effects and their ratios. We generated acceptability curves to represent the uncertainty in the cost-effectiveness estimates. These present the probability that each of the four strategies are cost effective for a range of values that a decision maker may be willing to pay for each additional QALY generated.
Results
The main characteristics of patients enrolled in the CARDERA trial are summarized in Table 1. The four treatment groups enrolled between 115 and 119 patients; their mean disease durations were ≤5 months and their mean ages were between 54 and 55 years. Between 23% and 37% were male and ≥94% were white Europeans. They initially had high disease activities (mean DAS28 scores ≥5.6) and disability levels were high (mean HAQ scores ≥1.4).
Characteristics of patients enrolled in the CARDERA trial
| . | MTX (n = 117) . | MTX + CIC (n = 119) . | MTX + steroid (n = 115) . | Triple therapy (n = 116) . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Mean . | s.d. . | Mean . | s.d. . | Mean . | s.d. . | Mean . | s.d. . |
| Comparison of groups showing baseline patient characteristics | ||||||||
| Gender, male, % | 37 | 33 | 23 | 32 | ||||
| Ethnicity, white European, % | 97 | 96 | 94 | 97 | ||||
| Age, years | 54 | (13) | 54 | (14) | 54 | (10) | 55 | (12) |
| Disease duration, months | 2.8 | (4.3) | 4.1 | (5.6) | 5.1 | (5.7) | 3.3 | (5.0) |
| DAS28 | 5.72 | (1.24) | 5.93 | (1.26) | 5.82 | (1.33) | 5.61 | (1.29) |
| HAQ | 1.48 | (0.66) | 1.66 | (0.67) | 1.61 | (0.68) | 1.56 | (0.68) |
| EQ-5D | 0.46 | (0.31) | 0.39 | (0.35) | 0.40 | (0.31) | 0.43 | (0.33) |
| . | MTX (n = 117) . | MTX + CIC (n = 119) . | MTX + steroid (n = 115) . | Triple therapy (n = 116) . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Mean . | s.d. . | Mean . | s.d. . | Mean . | s.d. . | Mean . | s.d. . |
| Comparison of groups showing baseline patient characteristics | ||||||||
| Gender, male, % | 37 | 33 | 23 | 32 | ||||
| Ethnicity, white European, % | 97 | 96 | 94 | 97 | ||||
| Age, years | 54 | (13) | 54 | (14) | 54 | (10) | 55 | (12) |
| Disease duration, months | 2.8 | (4.3) | 4.1 | (5.6) | 5.1 | (5.7) | 3.3 | (5.0) |
| DAS28 | 5.72 | (1.24) | 5.93 | (1.26) | 5.82 | (1.33) | 5.61 | (1.29) |
| HAQ | 1.48 | (0.66) | 1.66 | (0.67) | 1.61 | (0.68) | 1.56 | (0.68) |
| EQ-5D | 0.46 | (0.31) | 0.39 | (0.35) | 0.40 | (0.31) | 0.43 | (0.33) |
| . | Mean . | 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . |
|---|---|---|---|---|---|---|---|---|
| Mean costs and QALYs for complete case and imputed datasets | ||||||||
| Complete case analysis (10 000 bootstraps) | ||||||||
| Year 1 costs | 3854 | 3390 | 3250 | 3159 | ||||
| Year 2 costs | 3649 | 3439 | 3107 | 3044 | ||||
| Total costs, £ | 7503 | 6251, 8754 | 6829 | 5640, 8018 | 6357 | 5548, 7165 | 6203 | 5598, 6808 |
| Year 1 QALYs | 0.759 | 0.673 | 0.737 | 0.823 | ||||
| Year 2 QALYs | 0.480 | 0.420 | 0.418 | 0.497 | ||||
| Total QALYs | 1.238 | 1.148, 1.329 | 1.093 | 0.963, 1.224 | 1.154 | 1.037, 1.272 | 1.320 | 1.215, 1.425 |
| Multiple imputation (100 imputed datasets × 5000 bootstraps) | ||||||||
| Cost, £ | 7425 | 6469, 8381 | 6783 | 5834, 7733 | 6617 | 5841, 7393 | 6499 | 5722, 7275 |
| QALYs | 1.148 | 1.051, 1.246 | 1.052 | 0.936, 1.167 | 1.097 | 0.998, 1.196 | 1.257 | 1.165, 1.350 |
| . | Mean . | 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . |
|---|---|---|---|---|---|---|---|---|
| Mean costs and QALYs for complete case and imputed datasets | ||||||||
| Complete case analysis (10 000 bootstraps) | ||||||||
| Year 1 costs | 3854 | 3390 | 3250 | 3159 | ||||
| Year 2 costs | 3649 | 3439 | 3107 | 3044 | ||||
| Total costs, £ | 7503 | 6251, 8754 | 6829 | 5640, 8018 | 6357 | 5548, 7165 | 6203 | 5598, 6808 |
| Year 1 QALYs | 0.759 | 0.673 | 0.737 | 0.823 | ||||
| Year 2 QALYs | 0.480 | 0.420 | 0.418 | 0.497 | ||||
| Total QALYs | 1.238 | 1.148, 1.329 | 1.093 | 0.963, 1.224 | 1.154 | 1.037, 1.272 | 1.320 | 1.215, 1.425 |
| Multiple imputation (100 imputed datasets × 5000 bootstraps) | ||||||||
| Cost, £ | 7425 | 6469, 8381 | 6783 | 5834, 7733 | 6617 | 5841, 7393 | 6499 | 5722, 7275 |
| QALYs | 1.148 | 1.051, 1.246 | 1.052 | 0.936, 1.167 | 1.097 | 0.998, 1.196 | 1.257 | 1.165, 1.350 |
CIC: ciclosporin; DAS28: 28-joint DAS; QALYs: quality-adjusted life-years.
Characteristics of patients enrolled in the CARDERA trial
| . | MTX (n = 117) . | MTX + CIC (n = 119) . | MTX + steroid (n = 115) . | Triple therapy (n = 116) . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Mean . | s.d. . | Mean . | s.d. . | Mean . | s.d. . | Mean . | s.d. . |
| Comparison of groups showing baseline patient characteristics | ||||||||
| Gender, male, % | 37 | 33 | 23 | 32 | ||||
| Ethnicity, white European, % | 97 | 96 | 94 | 97 | ||||
| Age, years | 54 | (13) | 54 | (14) | 54 | (10) | 55 | (12) |
| Disease duration, months | 2.8 | (4.3) | 4.1 | (5.6) | 5.1 | (5.7) | 3.3 | (5.0) |
| DAS28 | 5.72 | (1.24) | 5.93 | (1.26) | 5.82 | (1.33) | 5.61 | (1.29) |
| HAQ | 1.48 | (0.66) | 1.66 | (0.67) | 1.61 | (0.68) | 1.56 | (0.68) |
| EQ-5D | 0.46 | (0.31) | 0.39 | (0.35) | 0.40 | (0.31) | 0.43 | (0.33) |
| . | MTX (n = 117) . | MTX + CIC (n = 119) . | MTX + steroid (n = 115) . | Triple therapy (n = 116) . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Mean . | s.d. . | Mean . | s.d. . | Mean . | s.d. . | Mean . | s.d. . |
| Comparison of groups showing baseline patient characteristics | ||||||||
| Gender, male, % | 37 | 33 | 23 | 32 | ||||
| Ethnicity, white European, % | 97 | 96 | 94 | 97 | ||||
| Age, years | 54 | (13) | 54 | (14) | 54 | (10) | 55 | (12) |
| Disease duration, months | 2.8 | (4.3) | 4.1 | (5.6) | 5.1 | (5.7) | 3.3 | (5.0) |
| DAS28 | 5.72 | (1.24) | 5.93 | (1.26) | 5.82 | (1.33) | 5.61 | (1.29) |
| HAQ | 1.48 | (0.66) | 1.66 | (0.67) | 1.61 | (0.68) | 1.56 | (0.68) |
| EQ-5D | 0.46 | (0.31) | 0.39 | (0.35) | 0.40 | (0.31) | 0.43 | (0.33) |
| . | Mean . | 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . |
|---|---|---|---|---|---|---|---|---|
| Mean costs and QALYs for complete case and imputed datasets | ||||||||
| Complete case analysis (10 000 bootstraps) | ||||||||
| Year 1 costs | 3854 | 3390 | 3250 | 3159 | ||||
| Year 2 costs | 3649 | 3439 | 3107 | 3044 | ||||
| Total costs, £ | 7503 | 6251, 8754 | 6829 | 5640, 8018 | 6357 | 5548, 7165 | 6203 | 5598, 6808 |
| Year 1 QALYs | 0.759 | 0.673 | 0.737 | 0.823 | ||||
| Year 2 QALYs | 0.480 | 0.420 | 0.418 | 0.497 | ||||
| Total QALYs | 1.238 | 1.148, 1.329 | 1.093 | 0.963, 1.224 | 1.154 | 1.037, 1.272 | 1.320 | 1.215, 1.425 |
| Multiple imputation (100 imputed datasets × 5000 bootstraps) | ||||||||
| Cost, £ | 7425 | 6469, 8381 | 6783 | 5834, 7733 | 6617 | 5841, 7393 | 6499 | 5722, 7275 |
| QALYs | 1.148 | 1.051, 1.246 | 1.052 | 0.936, 1.167 | 1.097 | 0.998, 1.196 | 1.257 | 1.165, 1.350 |
| . | Mean . | 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . | Mean . | 95% CI . |
|---|---|---|---|---|---|---|---|---|
| Mean costs and QALYs for complete case and imputed datasets | ||||||||
| Complete case analysis (10 000 bootstraps) | ||||||||
| Year 1 costs | 3854 | 3390 | 3250 | 3159 | ||||
| Year 2 costs | 3649 | 3439 | 3107 | 3044 | ||||
| Total costs, £ | 7503 | 6251, 8754 | 6829 | 5640, 8018 | 6357 | 5548, 7165 | 6203 | 5598, 6808 |
| Year 1 QALYs | 0.759 | 0.673 | 0.737 | 0.823 | ||||
| Year 2 QALYs | 0.480 | 0.420 | 0.418 | 0.497 | ||||
| Total QALYs | 1.238 | 1.148, 1.329 | 1.093 | 0.963, 1.224 | 1.154 | 1.037, 1.272 | 1.320 | 1.215, 1.425 |
| Multiple imputation (100 imputed datasets × 5000 bootstraps) | ||||||||
| Cost, £ | 7425 | 6469, 8381 | 6783 | 5834, 7733 | 6617 | 5841, 7393 | 6499 | 5722, 7275 |
| QALYs | 1.148 | 1.051, 1.246 | 1.052 | 0.936, 1.167 | 1.097 | 0.998, 1.196 | 1.257 | 1.165, 1.350 |
CIC: ciclosporin; DAS28: 28-joint DAS; QALYs: quality-adjusted life-years.
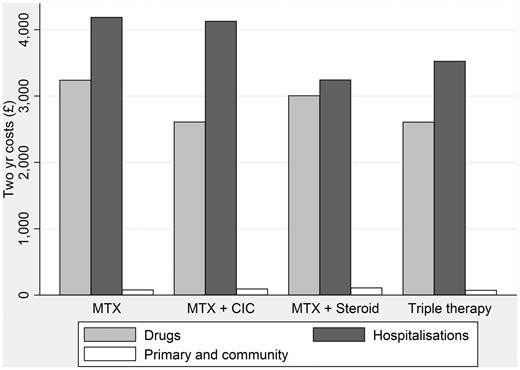
Two-year costs are summarized in Fig. 1, grouped by those incurred during hospital attendances, drug costs (including study drugs) and primary and community care costs for each of the treatment strategies. Primary care costs were negligible for all groups. Drug costs were lowest in the MTX/ciclosporin and triple therapy treatment arms. Mean hospital costs were lowest for the MTX/prednisolone and triple therapy arms. Hospital costs were substantially higher in the MTX monotherapy and the MTX/ciclosporin arms.
The mean costs and effects with associated bootstrap CIs for both the complete cases and imputed datasets are shown in Table 1. Up to 10 000 bootstrap replications were tested for the complete cases; no differences were found between these results and those for 5000 replications. Therefore 5000 replications were made for the imputed datasets, of which 100 were generated. The imputation of missing data lowers the estimate of mean QALYs for all four treatment strategies. The costs increase for MTX/prednisolone and triple therapy, while they decrease marginally for MTX monotherapy and for MTX/ciclosporin. QALYs were consistent between the complete case and multiple imputation analyses.
Triple therapy was the least costly and most effective and therefore dominated all other treatment strategies. Of the remaining strategies, MTX/ciclosporin was dominated by MTX/prednisolone. MTX monotherapy was more effective and more costly than MTX/prednisolone, with an incremental cost-effectiveness ratio of £13 700 and £15 600 using the complete cases and imputed data analyses, respectively. Results were consistent across years 1 and 2 except that MTX/prednisolone generated marginally lower QALYs than MTX/ciclosporin in year 2.
The cost-effectiveness acceptability curve for the imputed data analysis is shown in supplementary Fig. S1, available at Rheumatology Online. At a willingness to pay for each QALY gained of zero, the probability that triple therapy is the most cost effective of the four strategies is ∼0.5. This is equivalent to the probability that this strategy is the lowest cost. At positive values for a QALY in the range considered typical for the UK (£20 000–£30 000) this probability increase to >0.9. There were no significant differences in the plot when considering only complete cases.
Discussion
This economic analysis of the CARDERA trial demonstrates that treating early active RA patients intensively with triple therapy combining two DMARDs and short-term glucocorticoids is cost effective. Triple therapy achieved the lowest mean costs and highest mean QALY benefits and dominated monotherapy and double therapy treatment options. Our evaluation was limited to the 2-year within-trial period. We considered including a decision analytic model to extrapolate costs and benefits over longer time periods, however, drivers of any differences like withdrawal rates from DMARDs and assumptions about using high-cost biologics are too poorly defined for such an approach.
Our findings support the modelling used by NICE to develop RA guidelines; these advocate combination DMARDs and glucocorticoids in early RA. They reflect results in keeping with previous trials that examined this issue using individual patient-level resource use data. The COBRA trial reported lower mean costs per patient over 56-weeks follow-up using step-down prednisolone, MTX and SSZ ($5519) compared with SSZ alone ($6511); clinical, radiological and functional outcomes significantly favoured combination treatment [11]. The BeST trial reported better HRQoL improvements when initial combination therapy incorporated infliximab, but when the analysis was restricted to health care costs alone, initial combination therapy incorporating glucocorticoids was preferable [10]. The cost-effectiveness of intensive treatment in early RA was also shown by the TICORA trial. Although not strictly evaluating combination DMARDs vs DMARD monotherapy, TICORA reported greater effectiveness without additional cost with an intensive strategy escalating DMARD dosing according to patient response over 18 months [12].
The economic case for intensive treatment with conventional DMARDs was reviewed by Fautrel [18]. He showed RA medical costs increase with increasing disability levels and delaying disability by better disease control is therefore economically beneficial, particularly when using low-cost conventional DMARDs and glucocorticoids. Our findings strengthen the economic case for intensive combination DMARDs and short-term glucocorticoids. Although early biologics may be equally clinically effective as intensive DMARDs in early RA, their high costs indicate that they should be limited to individuals unresponsive to initial combination DMARDs. This was shown in a recent systematic review, as therapeutic escalation to anti-TNF represented a cost-effective strategy when synthetic DMARDs proved insufficient [19].
Our analysis has several limitations. It used data from patients enrolled >10 years ago. Although mean disease durations in patients enrolled in CARDERA were low and similar to those reported from English centres treating patients routinely between 2002 and 2007 [20], new English guidance of starting treatment within 6 weeks of referral might lead to patients starting MTX slightly earlier. At the same time, the maximal target dose of MTX recommended in recent trials has increased to 30 mg/week. These changes could have an additive effect. As a consequence, it is possible that CARDERA could potentially underestimate the effectiveness of MTX monotherapy. Current treatment regimens do not usually centre on high-dose prednisolone or ciclosporin, so the generalizability of our findings to more widely used treatment regimens is therefore uncertain. The range of biomarkers used to identify patients in CARDERA was limited; using ACPA and other prognostic markers to select patients for intensive therapy may enhance its impact. Finally, we only analysed health service costs. Including loss of work and other societal costs may have enhanced the apparent benefits of intensive treatment, although there may be difficulties in interpreting cost-effectiveness results that adopt this broader perspective. We conclude that intensive treatment of early RA with combination DMARDs and short-term glucocorticoids is both clinically and cost effective, supporting current management guidelines.
Combination DMARDs with glucocorticoids are likely to be clinically effective in early RA.
The cost-effectiveness of intensive early treatment of RA in the UK is not well known.
Trial-based analysis suggests triple therapy is more effective and less costly than other treatments for RA.
Acknowledgements
The views expressed are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Funding: This work presents independent research funded by the National Institute for Health Research under its Research for Patient Benefit (RfPB) Programme (grant reference number PB-PG-1208-18256). It was also supported by Arthritis Research UK (grant reference number 19739 to I.C.S.).
Disclosure statement: The authors have declared no conflicts of interest.




Comments