-
PDF
- Split View
-
Views
-
Cite
Cite
Jack D Edinger, Simon Beaulieu-Bonneau, Hans Ivers, Bernard Guay, Lynda Bélanger, Bryan Simmons, Charles M Morin, Association between insomnia patients’ pre-treatment characteristics and their responses to distinctive treatment sequences, Sleep, Volume 45, Issue 1, January 2022, zsab245, https://doi.org/10.1093/sleep/zsab245
Close - Share Icon Share
Abstract
It is common to provide insomnia patients a second treatment when the initial treatment fails, but little is known about optimal treatment sequences for different patient types. This study examined whether pre-treatment characteristics/traits predict optimal treatment sequences for insomnia patients.
A community sample of 211 adults (132 women; Mage = 45.6 ± 14.9 years) with insomnia were recruited. Patients were first treated with behavioral therapy (BT) or zolpidem (Zol). Non-remitting BT recipients were randomized to a second treatment with either Zol or cognitive therapy; non-remitting Zol recipients underwent BT or Trazodone as a second treatment. Remission rates were assessed at the end of the first and second 6-week treatments. We then compared the remission rates of dichotomous groups formed on the basis of gender, age, pretreatment scores on SF36 and Multidimensional Fatigue Scale, the presence/absence of psychiatric/medical comorbidities or pain disorders, and mean subjective sleep duration and efficiency within and across treatment sequences.
Lower remission rates were noted for those: with a pain disorder, poor mental health perceptions, high MFI fatigue scores, and lower sleep times and efficiencies. Patients with a pain disorder responded best to the BT-to-Zol sequence, whereas patients with more mental impairment, severe fatigue, short sleep, and low sleep efficiency responded poorly to treatment starting with BT.
Pain, fatigue, poor mental health status, and subjective sleep duration and efficiency all affect response to different insomnia treatment sequences. Findings may guide clinicians in matching insomnia treatments to their patients.
ClinicalTrials.gov Identifier: NCT01651442, Protocol version 4, April 20, 2011, registered June 26, 2012, https://clinicaltrials.gov/ct2/show/NCT01651442?rslt=With&type=Intr&cond=Insomnia&cntry=US&state=US%3ACO&city=Denver&age=12&draw=2&rank=1.
Both psychological/behavioral and pharmacological therapies have proven efficacy for insomnia management. Yet no one therapy is universally effective so patients may be provided an alternate treatment when the initial treatment fails. This study examined pre-treatment characteristics and traits of patients that may predict what treatment sequences prove effective for them. Findings suggest that factors such as patients’ perceived levels of mental health, levels of daytime fatigue, reports of a pain disorder, and subjective measures of total sleep time and sleep consolidation derived from commonly used sleep diaries may affect the outcomes of insomnia treatment sequences that are selected. The results provide some initial guidance to clinicians and highlight some easily considered patient characteristics that can be considered when selecting insomnia treatment sequences for distinctive types of insomnia patients.
Introduction
A number of pharmacological [1] and psychological/behavioral therapies [2] have proven efficacious for insomnia management, yet no single treatment is universally effective across all insomnia patients. As a consequence, a number of studies have been conducted to identify factors that can be used to predict patients’ responses to these therapies. Studies of prescription sleep aids have shown that patients taking eszopiclone responded best if their pre-treatment values of sleep latency and wake time fell below 75 min and 80 min respectively [3], whereas later bedtimes and shorter times between hypnotic administration and the subsequent AM wake time predicted greater sleep satisfaction among a group of patients using either benzodiazepines or benzodiazepine receptor agonists [4]. Studies of the psychological/behavioral insomnia therapies have shown that patients who have strong unhelpful beliefs about sleep, high distress about their insomnia, longer sleep onset latencies, longer total sleep times, higher insomnia severity, accurate sleep perceptions, and no psychiatric comorbidities prior to treatment, show the best outcomes with these interventions [5–10]. In addition, factors such as treatment acceptance and adherence as well as early response during the course of treatment have been shown to predict positive outcomes with these therapies [11–14]. Collectively, findings from these studies can be useful to clinicians when deciding upon a specific treatment for their insomnia patients and identifying patients who may need extra treatment attention.
However, in the clinical setting, treatment assignment is often guided by factors such as patient and clinician preferences as well as pragmatic considerations such as the accessibility of specific insomnia therapies. As such, many insomnia patients may not be initially provided their optimal insomnia therapy and, thus, show only a partial or no response to the initial treatment they receive. In such circumstances, it is common clinical practice to subsequently switch the patient to an alternate therapy in order to achieve a better outcome than the outcome derived from the initially provided therapy. Such sequencing of treatments can entail switching treatment modalities (i.e. medication to psychological/behavioral treatment and vice versa) or changing treatment types within a particular modality (e.g. switching from one medication to another or sequentially using different types of psychological/behavioral treatments). Unfortunately, research designed to predict optimal treatment sequences for patients who vary in their demographic characteristics and presenting clinical features has been lacking in the insomnia literature. Thus, clinicians’ choices of treatment sequences for the insomnia patients they encounter are guided primarily by their past experiences and intuitions.
Given these considerations, we conducted this study to determine if demographic or other easily assessable presenting symptoms of insomnia patients predict the sequences of insomnia therapies that produce the best treatment outcomes. Specifically, we examined whether patient’s genders, ages, pain conditions, comorbidities, pre-therapy average sleep times, and scores on commonly used psychometric instruments are predictive of treatment sequences that produce optimal treatment outcomes for insomnia patients. The data for this study were obtained from our previously published study [15] that compared four separate treatment sequences among a mixed sample of insomnia patients with and without comorbidities. Our previous study showed that first-stage treatment with behavioral therapy and zolpidem produced equivalent insomnia remission rates, but adding a second-stage treatment increased the overall remission rates observed. Yet neither our prior study nor other insomnia trials have examined whether patients’ pre-treatment characteristics or traits might predict what insomnia treatment sequences prove optimal for them. As no studies examining predictors of optimal treatment sequences have previously been conducted, there currently is no guidance for hypotheses development. As such, our study objectives remained exploratory and, therefore, were expected to produce findings that could better inform future studies of this nature.
Methods
Study design
The study was a single-blinded, randomized, controlled trial conducted at two sites: Institut universitaire en santé mentale de Québec (Université Laval, Québec, Canada) and National Jewish Health, Denver, CO. The study protocol was approved by the local ethics committees, and all participants provided written informed consent at their first screening visit.
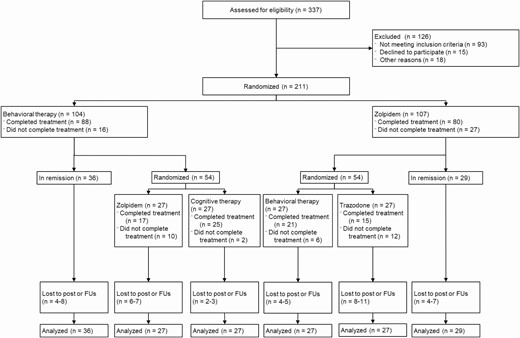
This project employed a Sequential Multiple Assignment Randomized Trial (SMART) design with treatments provided in two stages. Participants meeting selection criteria first completed a pre-treatment baseline assessment that included several psychometric instruments that are described below. They then were randomly assigned in a 1:1 ratio to behavioral therapy (BT) or zolpidem therapy (Zol), stratified by gender, age (<55 years vs. ≥55 years), and presence of a comorbid psychiatric disorder. Randomization was conducted at each site and allocations were concealed by using sequentially numbered sealed-envelopes opened only after patients met selection criteria and were ready for treatment. After the initial 6-weeks of therapy, patients meeting insomnia remission criteria were followed for the next 12 months on maintenance therapy while non-remitters were randomized (stratified by first randomization and comorbidity) to a second-stage treatment. Those who initially received BT were provided either Zol or cognitive therapy (CT) as their second-stage treatment whereas those who initially received Zol were randomly assigned either BT or Trazodone (Traz) as their second-stage therapy. Measurements were taken at baseline, the end of first- and second-stage therapies, and at follow-ups conducted 3, 6, and 12 months after the week-12 assessment. Figure 1 shows the overall study design and flow of participants through the various stages of the trial. For the purpose of the current study, only the treatment outcome data obtained at the end (i.e. weeks 5 and 6) of the first- and second-stage treatments were considered for analyses.
Figure is the Consort Diagram showing the flow of study participants completing various stages of the clinical trial.
Participants
A total of 211 adults with insomnia complaints were recruited from the community through media advertisements and from outpatient clinics. All individuals included in the study met the diagnostic criteria for an Insomnia Disorder as outlined in the Fifth Edition of the American Psychiatric Association’s Diagnostic and Statistical Manual [16]. Consistent with these criteria all study participants had a complaint of persistent (i.e. >3 months) difficulties initiating or maintaining sleep as reflected by a sleep onset latency or wake time after sleep onset >30 min 3 or more nights corroborated by 2 weeks of sleep diary monitoring. All enrollees also had an Insomnia Severity Index (ISI) total score > 10 that included a score ≥ 2 on either the interference or distress item of the screening ISI. Excluded were those having: (1) an untreated psychiatric disorder (e.g. major depression), (2) a lifetime diagnosis of any psychotic or bipolar disorder, (3) an imminent risk for suicide, (4) alcohol or drug abuse within the past year, (5) a terminal or progressive physical illness (e.g. cancer, COPD), or neurological degenerative disease (e.g. dementia), (6) current use of medications known to cause insomnia (e.g. steroids), (7) sleep apnea (apnea/hypopnea index > 15), restless legs syndrome, periodic limb movement during sleep (PLMS with arousal > 15 per hour), or a circadian rhythm sleep disorder (e.g. advanced sleep phase syndrome), (8) habitual bedtimes later than 2:00 AM or rising times later than 10:00 AM, and (9) >2 alcoholic beverages per day on a regular basis.
The sample obtained had a mean (SD) age of 45.6 (14.9) years and was comprised of 132 women and 79 men. Of the 211 enrolled, 187 (90.4%) were white. The sample had a mean (SD) educational level of 16.1 (3.3) years and had suffered from insomnia on average 13.2 (12.5) years.
Outcome measure
The primary study endpoint was the proportion of individuals achieving insomnia remission status, based on their scores obtained from the Insomnia Severity Index (ISI) [17, 18]. The ISI is a well-validated instrument that has proven sensitive to therapeutic changes [18]. For the purposes of this study, remission was defined as a mean ISI score < 8 for weeks 5 and 6 of each treatment stage, with none of these ISI scores > 10.
Other psychometric assessment instruments
In addition to the ISI, several other commonly used psychometric instruments were administered at baseline, post-treatment, and follow-up time points to provide measures of secondary outcome measures of interest. These included the Beck Depression Inventory-II (BDI-II) [19] to assess patients’ depression levels, the SF-36 Health Survey (SF-36) [20] to assess patients’ perceptions of their mental and physical health, and the 20-item Multidimensional Fatigue Inventory (MFI) [21] to assess patients’ fatigue levels. For the purpose of this investigation, only baseline scores of these instruments were used to examine how pre-treatment levels of depression, mental and physical health perceptions, and fatigue levels might moderate responses to the various insomnia treatment sequences included in this trial. The manners in which scores from these instruments were used for this purpose are described below.
Moderator variables
Our primary study objective was that of determining whether factors such as common demographic variables, the presence vs. absence of comorbidities, findings from sleep diaries, and scores from commonly used psychometric instruments might be useful in predicting patients’ optimal treatment sequences for achieving insomnia remission status. As described below, all moderators tested were considered as dichotomous variables to simplify study analyses and resultant clinical decision making.
Demographic variables
Both patients’ genders and ages were considered as moderators/ predictors in our analyses. In terms of gender, our patients were classified as male or female based on the gender they reported on our study’s intake questionnaires. Although patients enrolled in this study ranged in ages, the median age of our sample was 45 years. Hence, we dichotomized our sample into two age groups composed of those <45 years and those >45 years of age for the purposes of our planned analyses.
Psychiatric comorbidity and treatment
We also considered the possibility that the presence vs. absence of a psychiatric condition, patients’ perceptions of their general mental health statuses, and ongoing pharmacotherapy specifically for depression all could influence insomnia remission rates shown by the treatment sequences we tested. First, we used information from intake interviews and findings derived from the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) [22] to dichotomized our sample into those with and without psychiatric comorbidities. This was done to allow us to assess the effect of having any such comorbidity on remission rates observed. Secondly, we specifically tested whether pre-treatment levels of depression influenced the insomnia remission rates observed. To do so, we used scores participants obtained on the BDI-II [19] during their pre-treatment assessments to dichotomize our sample into to “moderate-high” and “low” depression groups. Those who had a BDI-II score at or above the sample median score = 14 were placed into the moderate-high depression group and those who had a BDI-II score < 14 were placed in the low depression group for outcome comparisons. Thirdly, we assessed the effects of our patients’ personal mental health perceptions on their remission rates in response to the treatments they were provided. To do so we dichotomized the sample into those having better or poorer personal mental health perceptions using the median score = 42 obtained by the sample on the mental composite scale of the SF-36 [20] administered prior to treatment. Those with scores < 42 were classified as having poor mental health perceptions, whereas those with scores > 42 were classified as having good mental health perceptions for the purpose of treatment outcome comparisons. Finally, we compared the remission rates shown by those who were and were not taking antidepressants during their study participation.
Medical comorbidities
Information provided by patients during intake interviews and physical exams conducted at screening was used to dichotomize our sample into groups with and without medical comorbidities (e.g. hypertension, diabetes, arthritis, etc.) to compare the insomnia remission rates of these two groups in response to the various treatment sequences employed. The information from physical exam and intake interviews was also used to dichotomize our sample into those with and without a chronic pain syndrome. This was done to allow us to compare the remission rates of those with and without a pain disorder in response to the various insomnia treatment sequences tested.
Daytime fatigue
In addition, we considered whether pre-treatment measures of daytime fatigue might be predictive of optimal treatment sequences for achieving insomnia remission. The sample’s median total score on the MFI [21] was used to dichotomize our sample into subgroups with high (score > 53) and low (score < 53) fatigue. We then conducted analyses to test for subgroup differences in insomnia remission rates within and across treatments sequences.
Treatment acceptance/usefulness
Once informed of their respective treatment assignments, participants completed the Therapy Evaluation Questionnaire TEQ [23] to indicate their perceptions of and expectations for the treatment they were assigned. The TEQ is a 7 item instrument that includes 5 questions (rated on a 9-point scale) assessing respondents’ perceived usefulness and acceptance of their assigned treatment condition, willingness to recommend it to a friend, willingness to repeat the treatment, etc. The TEQ also includes two items assessing the quality of the therapeutic relationship. For the purpose of the current investigation only the two items focused on the perceived usefulness and acceptance of the treatment were considered of most interest. The sample was, thus, dichotomized into subgroups using the median cutoff score of 7 for each of these two TEQ items with those having scores less than the median score of 7 on each item comprising one subgroup and those with scores > 7 comprising the other. We then statistically compared remission rates between the subgroups formed for each item and within subgroups across the various treatment sequences.
Subjective sleep measures
Subjective estimates of sleep and wake times were obtained daily for specified time periods during the study using a web-based sleep diary system. The diary was accessible to each participant via standard computer interface or via smart phone. The web-based diary was designed to mimic the Consensus Sleep Diary [24] and solicited information about the participant’s bedtime, sleep onset latency, number and length of nocturnal awakenings, time of final waking, rising time, sleep quality ratings, and sleep medication use. For the purpose of this study, we derived mean values of total sleep time (TST) and sleep efficiency (SE) from the sleep diaries during the two-week pre-treatment baseline period. Using these data, we then dichotomized the sample first into subgroups of short (mean TST < 6 h) and longer (mean TST > 6 h) sleepers and then into subgroups having low (mean SE < 75%) and high (mean SE > 75%) SE. The <6 h cutoff for defining short sleep was chosen based on our previous study [25] showing this cutoff identifies those who show a poor response to cognitive–behavioral insomnia therapy. The SE dichotomy formed was based on a median split of the observed values of SE in our study sample. The remission rates produced by each treatment sequence were then compared in the short and long sleepers and in the poor and good sleepers.
Treatments
Psychological therapy
The first-stage psychological therapy consisted of behavioral therapy (BT), which included sleep restriction [26] and stimulus control procedures [27]. These validated procedures are designed to strengthen homeostatic sleep drive, consolidate sleep via reducing time in bed, establishing a regular sleep schedule, and eliminating non-sleeping activities from the bedroom (i.e. using electronics, watching television, etc.). The second-stage psychological treatment consisted of cognitive therapy (CT), which included constructive worry [28] to reduce sleep disruptive mental activity in bed and strategies such as the use of thought records, recording automatic thoughts, Socratic questioning, and behavioral experiments [29, 30] to alter sleep- and mood-disruptive cognitions (i.e. thoughts, beliefs).
Medication
The first-stage medication treatment involved zolpidem (Zol), sublingual, 5 to 10 mg, taken nightly at bedtime. Zolpidem was selected as a first-stage therapy because of its documented efficacy and because it is among the most commonly prescribed medications for insomnia [1, 31]. All participants started with an initial dose of 5 mg, which was titrated up to 10 mg based on therapeutic response, side effects, and patient’s age and gender (as per FDA recommendations, dosage was limited to 5 mg in women). The second-stage pharmacotherapy consisted of trazodone (Traz; 50–150mg), taken 30 min before bedtime. We chose trazodone because it is also among the most commonly prescribed medications for insomnia (off-label) [32, 33]. At the end of first-stage medication treatment, participants who did not remit received a final drug supply and a written withdrawal schedule. Those who achieved remission with medication provided as a first- or second-stage treatment stayed on medication through follow-ups. These patients used a similar discontinuation schedule at the end of the 12-month follow up. Those who wished to continue medication were referred to their primary care physician for further follow-ups.
Treatment implementation and monitoring
All four treatments were administered in the context of four, individual, consultation visits led by clinical psychologists (BT, CT) or physicians/physicians’ assistants (Zol, Traz) spread over a 6-week period. They took place at weeks 1, 2, 4, and 6 and lasted 50 min for psychological treatments and 20 min for medication treatments. In addition to the main content pertaining to each treatment modality, both first-stage treatments (BT and Zol) included generic sleep hygiene education concerning the impact of stimulants, alcohol, caffeine, exercise, and environmental factors on sleep. Clinicians used treatment manuals and received ongoing supervision during the study to standardize treatment administration. Participants’ compliance with treatment protocols (e.g. time spent in bed, use of medication) were monitored by treating clinicians via sleep diaries and pill-count. For more detail about the study design, participants, and treatments, the reader is referred to our previously published study description [15].
Statistical analyses
This study used an intent-to-treat analysis. To evaluate each treatment sequence while taking into account the nature of the SMART design (i.e. two randomizations, where the second is conditional on the response to the first), the analytic strategy was based on Nahum-Shani recommendations [34] to use “randomization weights”. Percentage of remission at post-treatment (stage 1 or 2, whichever first happened) according to (1) four treatment sequences, (2) moderator (baseline predictor), and (3) sequence × moderator interaction, were analyzed using a weighted logistic (binary outcome) generalized estimating equations model (WGEE [35]). A priori contrasts within the WGEE models were used to test significance for comparisons between and within sequences.
To further clarify the statistical approach, remission status was analyzed at post-treatment (stage 1 or 2) according to the four treatment sequences included. From a clinical point of view, it did not seem reasonable to produce two sets of analyses, one for the end of the first-stage treatment and the other for the end of the second-stage treatment, as clinicians do not select a treatment sequence based on the endpoint. Since the study was designed to determine how well baseline variables and NOT the outcome served as predictors, a strategy was devised to unify endpoints (end of stage 1 and end of stage 2 treatments) and to use the first endpoint at which remission was observed. Thus, for those who remitted during the first stage, the outcome retained was the one observed at the end of the first-stage treatment, while for those who failed to remit by the end of their first-stage treatment, the preferred outcome was the one observed at the end of the second-stage treatment. This approach seemed most sensible since, in clinical practice, patients who achieve insomnia remission by the end of their initial therapy are dismissed from treatment and do not receive a second treatment.
Available outcome data from all patients were included in the analyses. For those who remitted after their first treatment (post1), the observed outcome at post1 was included in both stage-2 sequences which followed their first randomized sequence. For example, if a patient experienced remission by the end of the first-stage BT intervention, this remission was counted in both BT+ZOL and BT+CT sequences, but randomization weights were used to avoid counting this patient’s data as two patients. For those who failed to reach insomnia remission by the end of the first-stage treatment, the observed outcome (remission vs. non-remission) at the end of the second-stage treatment was included for the relevant stage 1–stage 2 sequence. Finally, for those who dropped out of the study during the trial, the last available weekly data point was included as the outcome. If attrition was observed during stage-1, it was treated like a stage-1 outcome for determining remission status and if attrition occurred during stage-2 then the last data point during the stage-2 treatment was treated as the stage-2 outcome for determining remission.
Results
Demographic factors
Analyses showed that gender was not a significant predictor of the insomnia remission rates observed in response to the treatments provided in this trial. Overall the remission rates for women (43.9%) and men (45.0%) were fairly comparable and not significantly different from each other statistically (p = 0.87). Moreover, the remission rates produced by the four treatment sequences examined did not differ significantly within the female (p = 0.73) or male (p = 0.16) groups.
Examination of our two age groups showed similar remission rates for those under 45 years of age (46.7%) and those age 45 and older (41.6%); and these rates did not differ significantly (p = 0.48). However, a significantly (p = 0.01) greater proportion of those in the younger age group dropped out of treatment compared to those in the older age group (39.2% vs. 18%). There were no significant differences between the two age groups’ remission rates they displayed in response to any of the four treatment sequences (p’s = 0.19–0.54) provided. Furthermore, the remission rates produced by the various treatment sequences examined did not differ significantly within the younger (p = 0.84) or older (p = 0.11) age groups.
Psychiatric comorbidity
For the most part, the presence vs. absence of any psychiatric comorbidity made little difference in the remission rates observed except for the treatment sequence beginning with BT and followed by zolpidem. With this sequence, those with a psychiatric comorbidity showed a significantly (p = 0.02) lower remission rate that did those without such comorbidity (32.5% vs. 62.4%). However, more significant differences were found when the sample was dichotomized based on the SF36 mental composite score. Table 1 shows the remission rates for those with lower and higher mental composite scores. Overall, those falling in the lower scoring group, connoting poorer perceived mental health, had a significantly (p = 0.04) lower remission rate than did the higher scoring group (35.0% vs. 55.5%). This finding seemed largely attributable to the two treatment sequences in which BT was provided as the initial therapy. As can be seen from Table 1, differences in remission rates across treatment sequences were not significant within either the group having low or high SF36 mental composite scores. The group with lower mental composite scores connoting poorer personal mental health perceptions had significantly lower remission rates for the BT+Zol (28.6% vs. 64.2%; p = 0.01) and the BT+CT (26.5% vs. 55.8%; p = 0.03) than did the higher scoring group with better personal mental health perceptions. In contrast, no statistically significant differences were noted in the analyses that dichotomized the sample based on their BDI depression scores.
Dropout and remission (% and SE) according to treatment sequence and predictor
| Predictor name and subgroupings . | Dropout . | Remission . | Remission according to full sequence . | . | |||
|---|---|---|---|---|---|---|---|
| SF36 mental score . | Overall % . | Overall % . | BT+ZOL . | BT+CT . | ZOL+BT . | ZOL+TRA . | Test* . |
| Good mental health score > 42 (n = 141) | 20.6 (3.4) | 50.5 (4.3) | 64..2 (7.4) n = 34 | 55.8 (7.5) n = 34 | 42.3 (7.1) n = 35 | 39.5 (6.7) n = 38 | 5.95, p = 0.11 |
| Poor mental health score < 42 (n = 62) | 29.7 (5.9) | 35.0 (6.2) | 28.6 (10.0) n = 16 | 26.5 (9.5) n = 17 | 37.4 (10.2) n = 16 | 49.3 (12.0) n = 13 | 2.40, p = 0.49 |
| Between comparison | p = 0.18 | p = 0.04 | p = 0.01 | p = 0.03 | p = 0.70 | p = 0.47 | |
| Pain condition | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 169) | 23.2 (3.3) | 49.5 (3.9) | 50.9 (6.6) n = 46 | 53.7 (6.9) n = 40 | 46.0 (6.5) n = 41 | 47.4 (6.5) n = 42 | 0.67, p = 0.88 |
| Yes (n = 42) | 35.7 (7.4) | 23.8 (7.1) | 56.4 (16.4) n = 7 | 22.8 (9.1) n = 13 | 13.1 (7.3) n = 12 | 14.2 (7.9) n = 10 | 7.06, p = 0.07 |
| Between comparison | p = 0.13 | p = 0.01 | p = 0.76 | p = 0.02 | p = 0.01 | p = 0.02 | |
| Fatigue level | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| Low fatigue MFI < 53 (n = 103) | 16.6 (3.7) | 53.4 (5.1) | 70.2 (8.3) n = 26 | 50.4 (8.4) n = 28 | 50.8 (9.2) n = 22 | 41.1 (8.1) n = 27 | 6.18, p = 0.10 |
| High fatigue MFI ≥ 53 (n = 100) | 30.1 (4.6) | 38.0 (4.9) | 35.0 (8.5) n = 24 | 41.2 (9.1) n = 23 | 32.9 (7.3) n = 29 | 43.1 (8.5) n = 24 | 1.74, p = 0.63 |
| Between comparison | p = 0.02 | p = 0.03 | p = 0.007 | p = 0.46 | p = 0.13 | p = 0.87 | |
| Short sleeper < 6 h | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 96) | 15.3 (3.8) | 58.4 (5.2) | 71.0 (8.7) n = 23 | 64.0 (8.6) n = 27 | 48.5 (9.3) n = 21 | 48.7 (8.8) n = 25 | 3.65, p = 0.30 |
| Yes (n = 92) | 24.5 (4.6) | 32.1 (5.0) | 36.4 (8.3) n = 26 | 24.9 (7.1) n = 23 | 33.7 (8.3) n = 23 | 34.0 (8.6) n = 20 | 2.00, p = 0.57 |
| Between comparison | p = 0.10 | p < 0.001 | p = 0.009 | p = 0.002 | p = 0.23 | p = 0.23 | |
| Poor sleep efficiency < 75% | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 85) | 12.2 (3.6) | 57.1 (5.5) | 67.2 (9.5) n = 20 | 61.8 (8.9) n = 25 | 46.7 (9.5) n = 19 | 51.9 (9.4) n = 21 | 2.32, p = 0.51 |
| Yes (n = 103) | 26.4 (4.5) | 34.9 (4.9) | 41.6 (8.3) n = 28 | 29.3 (7.7) n = 24 | 35.8 (8.2) n = 26 | 33.3 (8.1) n = 25 | 1.83, p = 0.61 |
| Between comparison | p = 0.01 | p = 0.003 | p = 0.05 | p = 0.01 | p = 0.38 | p = 0.14 | |
| Predictor name and subgroupings . | Dropout . | Remission . | Remission according to full sequence . | . | |||
|---|---|---|---|---|---|---|---|
| SF36 mental score . | Overall % . | Overall % . | BT+ZOL . | BT+CT . | ZOL+BT . | ZOL+TRA . | Test* . |
| Good mental health score > 42 (n = 141) | 20.6 (3.4) | 50.5 (4.3) | 64..2 (7.4) n = 34 | 55.8 (7.5) n = 34 | 42.3 (7.1) n = 35 | 39.5 (6.7) n = 38 | 5.95, p = 0.11 |
| Poor mental health score < 42 (n = 62) | 29.7 (5.9) | 35.0 (6.2) | 28.6 (10.0) n = 16 | 26.5 (9.5) n = 17 | 37.4 (10.2) n = 16 | 49.3 (12.0) n = 13 | 2.40, p = 0.49 |
| Between comparison | p = 0.18 | p = 0.04 | p = 0.01 | p = 0.03 | p = 0.70 | p = 0.47 | |
| Pain condition | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 169) | 23.2 (3.3) | 49.5 (3.9) | 50.9 (6.6) n = 46 | 53.7 (6.9) n = 40 | 46.0 (6.5) n = 41 | 47.4 (6.5) n = 42 | 0.67, p = 0.88 |
| Yes (n = 42) | 35.7 (7.4) | 23.8 (7.1) | 56.4 (16.4) n = 7 | 22.8 (9.1) n = 13 | 13.1 (7.3) n = 12 | 14.2 (7.9) n = 10 | 7.06, p = 0.07 |
| Between comparison | p = 0.13 | p = 0.01 | p = 0.76 | p = 0.02 | p = 0.01 | p = 0.02 | |
| Fatigue level | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| Low fatigue MFI < 53 (n = 103) | 16.6 (3.7) | 53.4 (5.1) | 70.2 (8.3) n = 26 | 50.4 (8.4) n = 28 | 50.8 (9.2) n = 22 | 41.1 (8.1) n = 27 | 6.18, p = 0.10 |
| High fatigue MFI ≥ 53 (n = 100) | 30.1 (4.6) | 38.0 (4.9) | 35.0 (8.5) n = 24 | 41.2 (9.1) n = 23 | 32.9 (7.3) n = 29 | 43.1 (8.5) n = 24 | 1.74, p = 0.63 |
| Between comparison | p = 0.02 | p = 0.03 | p = 0.007 | p = 0.46 | p = 0.13 | p = 0.87 | |
| Short sleeper < 6 h | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 96) | 15.3 (3.8) | 58.4 (5.2) | 71.0 (8.7) n = 23 | 64.0 (8.6) n = 27 | 48.5 (9.3) n = 21 | 48.7 (8.8) n = 25 | 3.65, p = 0.30 |
| Yes (n = 92) | 24.5 (4.6) | 32.1 (5.0) | 36.4 (8.3) n = 26 | 24.9 (7.1) n = 23 | 33.7 (8.3) n = 23 | 34.0 (8.6) n = 20 | 2.00, p = 0.57 |
| Between comparison | p = 0.10 | p < 0.001 | p = 0.009 | p = 0.002 | p = 0.23 | p = 0.23 | |
| Poor sleep efficiency < 75% | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 85) | 12.2 (3.6) | 57.1 (5.5) | 67.2 (9.5) n = 20 | 61.8 (8.9) n = 25 | 46.7 (9.5) n = 19 | 51.9 (9.4) n = 21 | 2.32, p = 0.51 |
| Yes (n = 103) | 26.4 (4.5) | 34.9 (4.9) | 41.6 (8.3) n = 28 | 29.3 (7.7) n = 24 | 35.8 (8.2) n = 26 | 33.3 (8.1) n = 25 | 1.83, p = 0.61 |
| Between comparison | p = 0.01 | p = 0.003 | p = 0.05 | p = 0.01 | p = 0.38 | p = 0.14 | |
*Comparison of remission rates between sequences for each level of predictor.
Dropout and remission (% and SE) according to treatment sequence and predictor
| Predictor name and subgroupings . | Dropout . | Remission . | Remission according to full sequence . | . | |||
|---|---|---|---|---|---|---|---|
| SF36 mental score . | Overall % . | Overall % . | BT+ZOL . | BT+CT . | ZOL+BT . | ZOL+TRA . | Test* . |
| Good mental health score > 42 (n = 141) | 20.6 (3.4) | 50.5 (4.3) | 64..2 (7.4) n = 34 | 55.8 (7.5) n = 34 | 42.3 (7.1) n = 35 | 39.5 (6.7) n = 38 | 5.95, p = 0.11 |
| Poor mental health score < 42 (n = 62) | 29.7 (5.9) | 35.0 (6.2) | 28.6 (10.0) n = 16 | 26.5 (9.5) n = 17 | 37.4 (10.2) n = 16 | 49.3 (12.0) n = 13 | 2.40, p = 0.49 |
| Between comparison | p = 0.18 | p = 0.04 | p = 0.01 | p = 0.03 | p = 0.70 | p = 0.47 | |
| Pain condition | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 169) | 23.2 (3.3) | 49.5 (3.9) | 50.9 (6.6) n = 46 | 53.7 (6.9) n = 40 | 46.0 (6.5) n = 41 | 47.4 (6.5) n = 42 | 0.67, p = 0.88 |
| Yes (n = 42) | 35.7 (7.4) | 23.8 (7.1) | 56.4 (16.4) n = 7 | 22.8 (9.1) n = 13 | 13.1 (7.3) n = 12 | 14.2 (7.9) n = 10 | 7.06, p = 0.07 |
| Between comparison | p = 0.13 | p = 0.01 | p = 0.76 | p = 0.02 | p = 0.01 | p = 0.02 | |
| Fatigue level | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| Low fatigue MFI < 53 (n = 103) | 16.6 (3.7) | 53.4 (5.1) | 70.2 (8.3) n = 26 | 50.4 (8.4) n = 28 | 50.8 (9.2) n = 22 | 41.1 (8.1) n = 27 | 6.18, p = 0.10 |
| High fatigue MFI ≥ 53 (n = 100) | 30.1 (4.6) | 38.0 (4.9) | 35.0 (8.5) n = 24 | 41.2 (9.1) n = 23 | 32.9 (7.3) n = 29 | 43.1 (8.5) n = 24 | 1.74, p = 0.63 |
| Between comparison | p = 0.02 | p = 0.03 | p = 0.007 | p = 0.46 | p = 0.13 | p = 0.87 | |
| Short sleeper < 6 h | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 96) | 15.3 (3.8) | 58.4 (5.2) | 71.0 (8.7) n = 23 | 64.0 (8.6) n = 27 | 48.5 (9.3) n = 21 | 48.7 (8.8) n = 25 | 3.65, p = 0.30 |
| Yes (n = 92) | 24.5 (4.6) | 32.1 (5.0) | 36.4 (8.3) n = 26 | 24.9 (7.1) n = 23 | 33.7 (8.3) n = 23 | 34.0 (8.6) n = 20 | 2.00, p = 0.57 |
| Between comparison | p = 0.10 | p < 0.001 | p = 0.009 | p = 0.002 | p = 0.23 | p = 0.23 | |
| Poor sleep efficiency < 75% | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 85) | 12.2 (3.6) | 57.1 (5.5) | 67.2 (9.5) n = 20 | 61.8 (8.9) n = 25 | 46.7 (9.5) n = 19 | 51.9 (9.4) n = 21 | 2.32, p = 0.51 |
| Yes (n = 103) | 26.4 (4.5) | 34.9 (4.9) | 41.6 (8.3) n = 28 | 29.3 (7.7) n = 24 | 35.8 (8.2) n = 26 | 33.3 (8.1) n = 25 | 1.83, p = 0.61 |
| Between comparison | p = 0.01 | p = 0.003 | p = 0.05 | p = 0.01 | p = 0.38 | p = 0.14 | |
| Predictor name and subgroupings . | Dropout . | Remission . | Remission according to full sequence . | . | |||
|---|---|---|---|---|---|---|---|
| SF36 mental score . | Overall % . | Overall % . | BT+ZOL . | BT+CT . | ZOL+BT . | ZOL+TRA . | Test* . |
| Good mental health score > 42 (n = 141) | 20.6 (3.4) | 50.5 (4.3) | 64..2 (7.4) n = 34 | 55.8 (7.5) n = 34 | 42.3 (7.1) n = 35 | 39.5 (6.7) n = 38 | 5.95, p = 0.11 |
| Poor mental health score < 42 (n = 62) | 29.7 (5.9) | 35.0 (6.2) | 28.6 (10.0) n = 16 | 26.5 (9.5) n = 17 | 37.4 (10.2) n = 16 | 49.3 (12.0) n = 13 | 2.40, p = 0.49 |
| Between comparison | p = 0.18 | p = 0.04 | p = 0.01 | p = 0.03 | p = 0.70 | p = 0.47 | |
| Pain condition | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 169) | 23.2 (3.3) | 49.5 (3.9) | 50.9 (6.6) n = 46 | 53.7 (6.9) n = 40 | 46.0 (6.5) n = 41 | 47.4 (6.5) n = 42 | 0.67, p = 0.88 |
| Yes (n = 42) | 35.7 (7.4) | 23.8 (7.1) | 56.4 (16.4) n = 7 | 22.8 (9.1) n = 13 | 13.1 (7.3) n = 12 | 14.2 (7.9) n = 10 | 7.06, p = 0.07 |
| Between comparison | p = 0.13 | p = 0.01 | p = 0.76 | p = 0.02 | p = 0.01 | p = 0.02 | |
| Fatigue level | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| Low fatigue MFI < 53 (n = 103) | 16.6 (3.7) | 53.4 (5.1) | 70.2 (8.3) n = 26 | 50.4 (8.4) n = 28 | 50.8 (9.2) n = 22 | 41.1 (8.1) n = 27 | 6.18, p = 0.10 |
| High fatigue MFI ≥ 53 (n = 100) | 30.1 (4.6) | 38.0 (4.9) | 35.0 (8.5) n = 24 | 41.2 (9.1) n = 23 | 32.9 (7.3) n = 29 | 43.1 (8.5) n = 24 | 1.74, p = 0.63 |
| Between comparison | p = 0.02 | p = 0.03 | p = 0.007 | p = 0.46 | p = 0.13 | p = 0.87 | |
| Short sleeper < 6 h | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 96) | 15.3 (3.8) | 58.4 (5.2) | 71.0 (8.7) n = 23 | 64.0 (8.6) n = 27 | 48.5 (9.3) n = 21 | 48.7 (8.8) n = 25 | 3.65, p = 0.30 |
| Yes (n = 92) | 24.5 (4.6) | 32.1 (5.0) | 36.4 (8.3) n = 26 | 24.9 (7.1) n = 23 | 33.7 (8.3) n = 23 | 34.0 (8.6) n = 20 | 2.00, p = 0.57 |
| Between comparison | p = 0.10 | p < 0.001 | p = 0.009 | p = 0.002 | p = 0.23 | p = 0.23 | |
| Poor sleep efficiency < 75% | Overall % | Overall % | BT+ZOL | BT+CT | ZOL+BT | ZOL+TRA | Test* |
| No (n = 85) | 12.2 (3.6) | 57.1 (5.5) | 67.2 (9.5) n = 20 | 61.8 (8.9) n = 25 | 46.7 (9.5) n = 19 | 51.9 (9.4) n = 21 | 2.32, p = 0.51 |
| Yes (n = 103) | 26.4 (4.5) | 34.9 (4.9) | 41.6 (8.3) n = 28 | 29.3 (7.7) n = 24 | 35.8 (8.2) n = 26 | 33.3 (8.1) n = 25 | 1.83, p = 0.61 |
| Between comparison | p = 0.01 | p = 0.003 | p = 0.05 | p = 0.01 | p = 0.38 | p = 0.14 | |
*Comparison of remission rates between sequences for each level of predictor.
Likewise use vs. non-use of antidepressant medication did not seem to influence remission outcomes. A total of 31 participants (14.7% of the initial sample) were using antidepressants at baseline (the most common were escitalopram, n = 12, venlafaxine, n = 7, buproprion, n = 5, and sertraline, n = 4). The % of antidepressant users was very balanced between conditions at stage 1 (15/104 = 14.4% for the behavior therapy condition versus 16/107 = 14.9% for the Zolpidem condition). Antidepressant use at baseline was not found to be predictive of study dropout (user = 25.6% dropout rate vs. non-user = 25.7%, p = 0.99) nor overall probability of insomnia remission (user = 46.5% of remission versus non-user = 44.1%, p = 0.82).
Medical comorbidity
There were no significant findings related to the presence or absence of a medical comorbidity. Specifically, the analyses did not show any differences in remission rates between those who did and did not have a medical comorbidity nor did analyses show any significant differences in remission rates within those groups with and without medical comorbidities across the four different treatment sequences. Likewise, we did not find any significant differences within or across groups dichotomized based on their SF36 physical component scores. However, as indicated by the data in Table 1, the presence of a pain disorder did influence the remission rates obtained. Overall, those with a pain disorder reported a significantly (p = 0.01) lower remission rate than did those without a pain disorder (23.8% vs. 49.5%). In addition, those with a pain disorder had significantly lower remission rates with the BT+CT (p = 0.01), Zol+BT (p = 0.02), and Zol+Traz (p = 0.01) than did those without a pain disorder. Among those with a pain disorder, there also was a trend (p = 0.07) for the BT+Zol to produce higher remission rates than the other three treatment sequences.
Fatigue
Table 1 shows the findings concerning those found to have lower or higher levels of fatigue suggested by their MFI scores. As shown by this table, a significantly (p = 0.02) greater proportion of the higher fatigue group dropped out of treatment and a significantly (p = 0.03) lower proportion from this group achieved insomnia remission than did the lower fatigue group. The group differences in remission rates seemed largely due to their differential remission rates in the BT+Zol treatment sequence. In fact, the remission rate for the lower fatigue group was twice as high as that shown for the high fatigue group for this treatment sequence. There was also a trend (p = 0.10) for this treatment sequence to outperform the other three sequences in the lower fatigue group. In contrast, no notable differences in remission rates were observed across treatment sequences within the higher fatigue group.
Treatment acceptance/usefulness
The group that rated the treatment as more highly acceptable had a significantly (p = 0.009) higher overall remission rate that did those who rated their assigned treatment as less acceptable (51.9% vs. 31.9%). Results also showed that a significantly (p = 0.02) greater percentage of those who rated the treatment as more highly acceptable achieved insomnia remission with the BT+CT treatment sequence than did those who rated their assigned treatment as less acceptable (62.7% vs. 34.7%). There were no significant differences in remission rates noted across treatment sequences within either the lower or higher treatment acceptance groups. In addition, none of the analyses conducted to compare those who had lower or higher ratings of the usefulness of their assigned treatment showed significant differences across treatment sequences within groups or significant remission rate differences between groups for any of the treatment sequences.
Sleep diary
Table 1 also shows remission rates for groups dichotomized by their subjective sleep durations and sleep efficiencies. As can be seen, those who reported averaging less than 6 h of sleep per night had significantly lower remission rates than did those who averaged 6 h or more of sleep per night. These findings seemed largely due to the poorer response of the short sleeper group to treatment sequences that began with BT. There were no significant differences in remission rates across treatment sequences within either the short or longer sleeper groups.
Table 1 additionally shows the outcomes for groups classified on the basis of their subjective sleep efficiencies. As can be seen, a significantly higher proportion of those in the lower sleep efficiency group dropped out of treatment and a significantly lower proportion of this group achieved insomnia remission. Once again this finding appeared attributable to the poorer response of the low sleep efficiency group to the two treatment sequences beginning with BT. We did not find statistically significant differences in remission rates across treatment sequences within either the low or higher sleep efficiency group although those with higher sleep efficiencies seemed to have the highest remission rates if they began treatment with BT.
Summary of findings
Table 2 provides a summary of the between group differences in remission rates for those characteristics or traits that significantly affected treatment responses. As can be seen from the table, persons having those traits generally responded more poorly to the treatment sequences beginning with BT than did those without these traits. Only in the case of pain did those with a pain disorder show comparable remission rates to the BT+ZOL sequence as did those without a pain disorder. For the remaining characteristics listed in Table 2, no significant between-group differences were found in response to the treatment sequences beginning with ZOL.
Characteristics associated with significantly lower remission rates for those with these characteristics versus those without these characteristics across the treatment sequences
| . | Treatment sequences . | |||
|---|---|---|---|---|
| Trait . | BT+ZOL . | BT+CT . | ZOL+BT . | ZOL+TRA . |
| Low SF36 mental score ≤ 42 | + | + | ||
| Pain disorder | + | + | + | |
| MFI (fatigue) score ≥ 53 | + | |||
| Short sleeper: <6 h – sleep diary) | + | + | ||
| Sleep efficiency <75% – sleep diary | + | + | ||
| . | Treatment sequences . | |||
|---|---|---|---|---|
| Trait . | BT+ZOL . | BT+CT . | ZOL+BT . | ZOL+TRA . |
| Low SF36 mental score ≤ 42 | + | + | ||
| Pain disorder | + | + | + | |
| MFI (fatigue) score ≥ 53 | + | |||
| Short sleeper: <6 h – sleep diary) | + | + | ||
| Sleep efficiency <75% – sleep diary | + | + | ||
MFI – Multidimensional Fatigue Inventory; + signs indicate treatment sequences that produce higher remission rates when the characteristic/trait is absent as opposed to present.
Characteristics associated with significantly lower remission rates for those with these characteristics versus those without these characteristics across the treatment sequences
| . | Treatment sequences . | |||
|---|---|---|---|---|
| Trait . | BT+ZOL . | BT+CT . | ZOL+BT . | ZOL+TRA . |
| Low SF36 mental score ≤ 42 | + | + | ||
| Pain disorder | + | + | + | |
| MFI (fatigue) score ≥ 53 | + | |||
| Short sleeper: <6 h – sleep diary) | + | + | ||
| Sleep efficiency <75% – sleep diary | + | + | ||
| . | Treatment sequences . | |||
|---|---|---|---|---|
| Trait . | BT+ZOL . | BT+CT . | ZOL+BT . | ZOL+TRA . |
| Low SF36 mental score ≤ 42 | + | + | ||
| Pain disorder | + | + | + | |
| MFI (fatigue) score ≥ 53 | + | |||
| Short sleeper: <6 h – sleep diary) | + | + | ||
| Sleep efficiency <75% – sleep diary | + | + | ||
MFI – Multidimensional Fatigue Inventory; + signs indicate treatment sequences that produce higher remission rates when the characteristic/trait is absent as opposed to present.
Discussion
The current study was conducted to determine whether certain patient characteristics or traits affect response to distinct treatment sequences provided for treatment of insomnia disorder. Overall, results of the analyses conducted showed no statistically significant differences across the four treatment sequences examined for any of the characteristics or traits we considered. However, we did observe some trends that seem notable. Examination of mean remission rates showed that those with high SF36 Mental Scores (i.e. good personal mental health perceptions) showed higher insomnia remission rates to the treatment sequences beginning with BT than they did to those beginning with ZOL (p = 0.11). Also, comparison of remission rates across treatment sequences within the group with a pain disorder showed a trend (p = 0.07) toward differences between these sequences favoring the BT+ZOL sequence. Finally, we found a trend (p = 0.10) for remission rate differences among the treatment sequences favoring the BT+ZOL sequence over the other sequences within the group with low MFI measured fatigue. Nonetheless, these findings can only be considered suggestive and not conclusive at this juncture as larger studies of this nature would be needed to determine the significance of these trends.
In contrast, several of the characteristics/traits we considered did seem to affect insomnia treatment response. As summarized, in Table 2, patients with poorer mental health, the presence of a pain disorder, high daytime fatigue, subjective short sleep duration (<6 h per night), and low subjective sleep efficiency (i.e. sleep efficiency < 75%) all were prone to have significantly lower insomnia remission rates than were those without these characteristics/traits. For those with poor mental health perceptions, high daytime fatigue, subjective short sleep and low sleep efficiency the remission rate differences occurred with treatment sequences beginning with BT. For those with a pain disorder, the BT+ZOL sequence seemed optimal in that it produced the highest mean remission rate and fairly comparable remission rates for those with and without pain. Hence, the findings summarized in Table 2 should be useful in guiding treatment selection for patients with the traits and disorders examined.
Of the various findings reported, those concerning sleep duration and quality are particularly interesting given the recent research concerning objective short sleep duration. Previous research has shown that those with objective sleep disturbance or short sleep duration (<6 h/night) have a poorer response to cognitive–behavioral insomnia therapy (CBT-I) than do those with a more normal average sleep duration [5, 25, 36]. The results obtained herein complement these findings by showing that those with average subjective sleep times shown by commonly used sleep diaries had significantly lower remission rates in response to the treatments starting with BT than did patients with more normative subjective sleep times. These findings would imply that adherence to and/or benefit from a behavioral treatment regimen may be difficult for those with short sleep. It seems reasonable to speculate that the sleep restriction component of BT may be especially difficult to implement in those who are already running a marked sleep deficit. At any rate, these findings suggest that BT may not be a good starting point in treatment for those whose sleep diaries show a short and fragmented sleep pattern.
An additional important point to consider is that some of the treatment sequences resulted in long-term treatment with a pharmacological agent (ZOL or TRAZ). Psychological and behavioral therapies have been strongly recommended for insomnia management [2], and such therapies are now regarded by some as the first-line treatment of choice for all adults with insomnia disorder [37]. Moreover, concerns have been raised about long-term use of sedative hypnotics due to potential eventual medication tolerance, reduced efficacy with continual use, and their associated adverse outcomes (e.g. falls, traffic accidents) [38–43]. Nonetheless, not all patients respond to psychological and behavioral insomnia therapies and some long-term studies have shown that medications such as zolpidem can remain effective and safe over long periods [44, 45]. Furthermore, a recent small trial [46] showed that trazodone was superior to CBT-I for increasing total sleep time and reducing physiological hyperarousal as reflected by cortisol levels among insomnia patients with short objective sleep duration. Thus, pharmacotherapy may produce better outcomes for some patient groups. Yet admittedly more studies that consider various psychological/behavioral and pharmacological treatment sequences are needed to determine what treatment sequences work best for different patient types.
In considering the findings reported herein, it is important to consider a number of this study’s limitations. The study sample was comprised largely of well-educated white individuals. As such it is unclear whether the results obtained would apply to other ethnic groups or to those with lower educational levels. Additionally, we did not collect information about participants’ annual incomes so we were unable to examine the relationships between insomnia remission and participants’ socio-economic statuses. Admittedly the overall sample size was moderate resulting in a relatively small number of participants being included in the personnel characteristic/trait × treatment sequence “cells” of our statistical analyses. As such, we had limited statistical power to detect statistical differences for many of the analyses conducted. Given this consideration, it is perhaps not surprising that we did not detect statistically significant within-group differences across the treatment sequences considered. We also should note that we examined a limited number of patients’ pre-treatment characteristics/traits, and the dichotomies formed for study analyses in several cases were limited. For example, we did not examine whether specific types of mental health conditions, medical disorders, or pain conditions were more or less predictive of treatment responses. Finally, we considered a limited number of treatment sequences, and CBT-I, which is currently considered the first-line treatment for insomnia disorder, was included only in a decomposed fashion in one of the treatment sequences. As such, it is unclear how this treatment would have fared if presented in a conventional fashion. It is also unclear how other forms of hypnotic medications and psychological/behavioral therapies would have performed as measured by insomnia remission rates. Nonetheless, our findings seem important in showing the relative value of a selected set of treatment sequences for insomnia patients with certain comorbidities and types of sleep patterns. Thus, additional larger studies to test various treatment sequences with different patient subtypes seem warranted.
Funding
This study was funded by the National Institute of Mental Health, Grant #s R01MH091075 and MH091053.
Acknowledgments
The authors would like to thank Philips Respironics, Inc, for donating the Alice PDX polysomnographic recorders and software used at the National Jewish Health site in this project. The views expressed herein are exclusively those of the authors and do not reflect the views of the National Institute of Mental Health.
Disclosure Statement
Financial Disclosure. Dr. Edinger has received previous funding from Merck and donated sleep recording equipment from Philips-Respironics. Dr. Morin has received research support from Idorsia, Canopy Health, Eisai, Lallemand Health Solutions and served as a consultant for Eisai, Pear Therapeutics, Sunovion and Weight Watchers. He received royalties from Mapi Research Trust. The other authors have no disclosures relevant to this study.
Non-financial Disclosure. None.
References





Comments