-
PDF
- Split View
-
Views
-
Cite
Cite
Falak Sher, Reinhard Rößler, Nieske Brouwer, Veerakumar Balasubramaniyan, Erik Boddeke, Sjef Copray, Differentiation of Neural Stem Cells into Oligodendrocytes: Involvement of the Polycomb Group Protein Ezh2, Stem Cells, Volume 26, Issue 11, November 2008, Pages 2875–2883, https://doi.org/10.1634/stemcells.2008-0121
Close - Share Icon Share
Abstract
The mechanisms underlying the regulation of neural stem cell (NSC) renewal and maintenance of their multipotency are still not completely understood. Self-renewal of stem cells in general implies repression of genes that encode for cell lineage differentiation. Enhancer of zeste homolog 2 (Ezh2) is a Polycomb group protein involved in stem cell renewal and maintenance by inducing gene silencing via histone methylation and deacetylation. To establish the role of Ezh2 in the maintenance and differentiation of NSCs, we have examined the expression of Ezh2 in NSCs isolated from embryonic (embryonic day 14) mice during proliferation and differentiation in vitro. Our results show that Ezh2 is highly expressed in proliferating NSCs. In accordance with its suggested role as a transcription repressor, the expression of Ezh2 decreased when the NSCs differentiated into neurons and was completely suppressed during differentiation into astrocytes. Surprisingly, Ezh2 remained highly expressed in NSCs that differentiated into an oligodendrocytic cell lineage, starting from oligodendrocyte precursor cells (OPCs) up to the immature (premyelinating) oligodendrocyte stage. To further establish the role of Ezh2 in NSC differentiation, we silenced and induced overexpression of the Ezh2 gene in NSCs. High levels of Ezh2 in differentiating NSCs appeared to be associated with an increase in oligodendrocytes and a reduction in astrocytes, whereas low levels of Ezh2 led to completely opposite effects. The increase in the number of oligodendrocytes induced by enhanced expression of Ezh2 could be ascribed to stimulation of OPC proliferation although stimulation of oligodendrocyte differentiation cannot be excluded.
Disclosure of potential conflicts of interest is found at the end of this article.
Introduction
Proteins encoded by the Polycomb group (PcG) genes are known to play a crucial role in the gene expression program, which allows stem cells to maintain a pluripotent state but also allows for differentiation into more specialized phenotypes when signaled to do so (for reviews, see [1–3]). The PcG proteins were first described in Drosophila, where they repress the homeotic genes controlling segment identity in the developing embryo [4, 5]. The PcG proteins form multiple Polycomb repressive complexes (PRCs), the components of which are conserved from Drosophila to humans. Among the known types of PRCs, PRC1 and PRC2 are the best described and as yet are considered to be the most important ones. Whereas PRC2 is of importance for the initiation of gene repression, PRC1 is involved in the maintenance of gene repression. At the site of repression the PRC2 initiation complex binds to the PcG target genes; one of the components of PRC2, enhancer of zeste homolog 2 (Ezh2), then trimethylates lysine 27 of histone H3. Subsequently, PRC1 recognizes the trimethylated H3K27 (H3K27me3) sites and silences the target genes either by direct inhibition of the transcriptional machinery, by ubiquitilation of H2AK119, by chromatin compaction, and/or by recruitment of DNA methyltransferases [2, 3]. Recently, cohorts of developmental genes that are silenced by PRC1 and PRC2 in murine and human embryonic stem cells have been identified [6–8]; removal from the target genes or functional modification of the PRCs leads to expression of these genes and to cellular differentiation. Thus, it has been found that in the transition from an embryonic stem cell into a neural stem cell, concomitant with a reduction of H3K27me3, the transcript levels of, for instance, Olig1, 2, and 3, EGFrec, Nes, and Sox-9 increase [6]. During subsequent cellular differentiation, the composition and histone substrates of the PRCs change considerably, altering their mere repressive function [9].
In addition, in adult stem cells, such as hematopoietic and neural stem cells, the PcG proteins have been shown to play an important role in self-renewal, maintenance of pluripotency, and repression of differentiation. The strongest evidence for neural stem cells has been provided for the PcG protein Bmi-1 (a component of PRC1) [10–14]; Bmi1-deficient mice exhibit aberrant transformations coupled with acute neurological disorders and severe proliferation defects during hematopoiesis [15].
Recently, involvement of the PRC2-PcG protein, Ezh2, in the maintenance and the prevention of aging of hematopoietic stem cells has been demonstrated [16]. In addition, preliminary indications suggest that Ezh2 is also expressed in adult neural stem cells in the hippocampus [17] and subventricular zone (Allen Brain Atlas, http://www.brain-map.org).
In the present study, we have investigated the expression of Ezh2 in undifferentiated neural stem cells and during their differentiation into neurons, astrocytes, and oligodendrocytes to establish its involvement in the regulation of maintenance and differentiation of neural stem cells.
Materials and Methods
Animals
For all experiments, C57BL/6 mice that were housed under standard conditions with free access to food and water were used. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and regulations of the local Experimental Animal Committee.
Cell Cultures—Primary Murine Neural Stem Cell Culture
Neural stem cells (NSCs) were isolated from the telencephalon of C57/BL6 mouse embryos at embryonic day 14 (E14). Briefly, the telencephalon was cut into small pieces at room temperature and after mechanical trituration in ice-cold phosphate-buffered saline, the tissue was incubated with Accutase (Sigma-Aldrich, Zwijndrecht, The Netherlands, http://www.sigmaaldrich.com) for 15 minutes at 37°C. After repeated trituration, the cell suspension was passed through a cell strainer (70-mm pore size; BD Falcon, Franklin Lakes, NJ, http://www.bdbiosciences.com) and seeded (1–1.5 million cells) in T25 (Nunc, Roskilde, Denmark, http://www.nuncbrand.com) tissue culture flasks containing proliferation medium, which consisted of Neurobasal medium (Invitrogen, Breda, The Netherlands, http://www.invitrogen.com) supplemented with B27 (2%, Invitrogen), human recombinant epidermal growth factor (EGF) (20 ng/ml, Invitrogen), basic fibroblast growth factor (bFGF) (20 ng/ml; Invitrogen), GlutaMAX (1%; GIBCO, Grand Island, NY, http://www.invitrogen.com), Primocin (100 mg/ml), and heparin (5 mg/ml; Sigma-Aldrich) in a humidified 5% CO2/95% air incubator at 37°C. Within 3–5 days the cells grew as free-floating neurospheres and were dissociated and passaged with the use of Accutase. To induce the differentiation of NSCs into the three neural cell types, neurospheres were exposed to Accutase for 5–10 minutes and dissociated by triturating. Subsequently, the cells were plated in poly-l-lysine and laminin-coated chamber slides (Nunc), at approximately 15,000 cells per well in Neurobasal medium supplemented with B27 for 5 days at 5% CO2 and 37°C.
Primary Murine Astrocyte Culture
Mixed glial cultures were established as described previously [18]. In short, brains from postnatal C57/BL6 mice were rapidly isolated, and after removal of meninges the cortices were dissected in ice-cold medium A (Hanks' balanced salt solution supplemented with 1.5% glucose and 1.5% HEPES). Cortices were dissociated mechanically by trituration in medium A and subsequently by trypsinization for 20 minutes at 37°C in medium A supplemented with 0.25% trypsin and 1,000 U/ml DNase I. After inhibition of trypsin with medium A containing 0.1 mg/ml trypsin inhibitor (Sigma-Aldrich), 20% fetal calf serum (FCS), and 1,000 U/ml DNase I, the cells were centrifuged for 10 minutes at 800 rpm. Single-cell suspensions were seeded in T75 cm2 culture flasks (Greiner Bio-One, Alphen a/d Rijn, The Netherlands, http://www.gbo.com/en) containing Dulbecco's modified Eagle's medium (DMEM) containing 10% FCS, 1% glutamine, 1% sodium pyruvate, and Primocin. After 7–10 days in vitro, the mixed glial cultures were placed overnight on a shaker (150 rpm) at 37°C, and on the following day the nonadherent glial cells were removed. Next, astrocytic monolayers were carefully separated from the underlying adherent microglia by a mild trypsinization step and plated in new T75 cm2 tissue culture flasks. In the following weeks, the overnight shaking and trypsinization steps were repeated until pure astrocyte cultures (>98%) were obtained.
Primary Murine Oligodendrocyte Culture
T75 cm2 flasks containing mixed glial culture were shaken overnight at 37°C. Subsequently, the supernatant-containing detached cells were passed through a cell strainer (70-mm pore size; Falcon). After centrifugation at 2,000 rpm, the pellet was resuspended in culture medium and plated in uncoated culture dishes for 30 minutes. Microglia and astrocytes attached to the bottom of the culture dish, whereas oligodendrocyte precursor cells (OPCs) remained floating. In consecutive turns the floating cells were transferred from one culture dish to another until a pure (>95%) OPC culture was obtained. After centrifugation, the primary OPCs were cultured in SATO medium consisting of DMEM with additives: GlutaMAX (1%), putrescine (16 μg/ml; Sigma-Aldrich), thyroxin (400 ng/ml; Sigma-Aldrich), triiodothyronine (400 ng/ml; Sigma-Aldrich), progesterone (6.2 ng/ ml; Sigma-Aldrich), sodium selenite (5 ng/ml; Sigma-Aldrich), bovine serum albumin (100 μg/ml; Sigma-Aldrich), insulin (5 μg/ml; Sigma-Aldrich), transferrin (50 μg/ml; Sigma-Aldrich), B27 (2%), and Primocin (100 mg/ml). During the first 2 days of culture, SATO medium was supplemented with Sonic hedgehog (100 ng/ml; R&D Systems, Abington, U.K., http://www.rndsystems.com) and platelet-derived growth factor (PDGF)-α (10 ng/ml; R&D Systems). During subsequent culturing, the OPCs differentiated and maturated along standard stages: oligodendroblasts, immature oligodendrocytes, and mature oligodendrocytes. Thus, suspensions of each stage cell type were obtained by culturing cells in SATO medium for 24 hours (OPCs), 48 hours (oligodendroblasts), 1 week (immature oligodendrocytes), and 3 weeks (mature oligodendrocytes), respectively. Medium was refreshed after every 2nd day.
Primary Murine Cortical Neuronal Culture
Cultures of cortical neurons were established as described previously [19]. In brief, cortices of C57/BL6 embryonic day 17 embryos were excised and collected in ice-cold HBSS supplemented with 30% glucose. Meninges were removed, and brain tissue was dissociated by trituration in Neurobasal-B27 medium (supplemented with 0.4% glucose, 2 mM l-glutamine, and 0.01% penicillin/streptomycin) and filtered through a cell strainer. After one washing step, cells were seeded on poly-d-lysine (10 mg/ml)-coated six-well plates and maintained in Neurobasal/B27 medium for 7 days in a humidified atmosphere (5% CO2/95% air) at 37°C.
Oligodendrocyte Cell Lines
Three oligodendrocyte cell lines were used: Oli-neu, N19, and N20.1. N19 and N20.1 (kindly provided by Dr. M. J. Leroy, University Rene Descartes, Paris, France) were cultured on poly-l-lysine-coated tissue culture flasks at permissive temperature (34°C) in DMEM/Ham's F12 medium, supplemented with 10% FCS, 1% glutamine, and 1% penicillin/streptomycin. The Oli-neu cell line (kindly provided by Dr. J. Trotter, Johannes Gutenberg University of Mainz, Mainz, Germany) was also cultured on poly-l-lysine-coated cell culture flasks in SATO medium with 1% horse serum at 37°C. N19 and N20.1 differentiation was induced by culturing these cells at nonpermissive temperature (39°C) with 1% FCS; 1 mM dibutyryl cyclic AMP was added for differentiation induction of the Oli-neu cells.
Gene Transfection of NSCs
Overexpression of Ezh2 was induced by using the Ezh2 expression vector (Ezh2 in pCMV-SPORT6; Open Biosystems, Huntsville, AL, http://www.openbiosystems.com) and Ezh2 expression was silenced by using Ezh2 short hairpin silencing RNA (shRNA) plasmid DNA constructs targeting the following sequence: CCGGGCGTATAAAGACACCACCTAACTCGAGTTAGGTGGTGTCTTTATACGCTTTTTG in the coding region of the Ezh2 gene (MISSION DNA NM 007971; Sigma-Aldrich); five vectors for Ezh2 silencing were tested, of which three appeared to be similarly efficient and showed the same results. In this article results with one of the three effective silencing vectors are presented. All gene transfections were performed by using an electroporesis-based transfection protocol (Amaxa GmbH, Cologne, Germany, http://amaxa.com) specifically designed for the transfection of embryonic mouse NSCs by Amaxa. By using Accutase, neurospheres were dissociated and 3–5 × 106 cells were transfected with 10 mg of DNA of the overexpression vector or 3 mg of the shRNA plasmid DNA vector. For control transfections, the same vector containing DNA-encoding green fluorescent protein (eGFP) was used. After transfection, the NSCs were kept for 24 hours (shRNA) and 72 hours (pCMV-SPORT6) in a humidified 5% CO2-95% air incubator at 37°C in Neurobasal medium supplemented with B27 and 1% FCS. The transfection efficiency of this procedure for mouse NSCs amounted to 60–80% and results in the transient expression of the transfected gene lasting up to 10–12 days [20]. Ezh2 expression after transfection was established at the RNA level (with reverse transcription [RT]-polymerase chain reaction [PCR]) and at protein level (with Western blotting); subsequently, NSCs were plated in poly-l-lysin/laminin-coated chamber slides (Nunc) to study the differentiation pattern.
Western Blot Analysis
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and Western blot analysis were used to detect Ezh2 protein expression in undifferentiated NSCs and different neural cell types. After centrifugation cells were resuspended in radioimmunoprecipitation assay buffer along with a cocktail of protease inhibitors. Resuspensions were sonicated for 5 seconds and centrifuged for 10 minutes at 12,000 rpm and 4°C. Pellets were discarded, and supernatants were mixed with Laemmli buffer and boiled for 5 minutes. Proteins were separated for 1.5 hours on a 10% SDS-polyacrylamide gel electrophoresis apparatus (Bio-Rad, Hercules, CA, http://www.bio-rad.com). Subsequently, proteins were transferred to nitrocellulose membranes by using semidry transfer buffer (25 mM Tris, 150 mM glycine, and 10% [v/v] methanol) and 3 mA/cm2 current for 45–50 minutes. Nitrocellulose membranes were blocked with 3% milk in TBS-T (Tris-buffered saline-0.1% Tween 20) for 1 hour at room temperature. Membranes were probed overnight at 4°C by using mouse anti-Ezh2 antibody (catalog no. 612666; BD Transduction Laboratory, San Jose, CA, http://www.bdbiosciences.com) at 1:1,000 dilution in blocking solution and mouse anti-β-actin (ab6276; Abcam, Cambridge, U.K.) at 1:5,000 dilution. (The housekeeping gene β-actin was used for normalization of Ezh2 expression levels.) Membranes were washed four times (10 minutes each) with TBS-T. Primary Ezh2-antibody and anti-β-actin antibody were detected using horseradish peroxidase-conjugated anti-mouse IgG (code no. P0260; DAKO, Heverlee, Belgium, http://www.dako.com). Incubation with secondary antibody was performed for 1 hour at room temperature at 1:10,000 dilutions in blocking solution. After four washes in TBS-T, protein signals were detected by an enhanced chemiluminescence detection system (Amersham Biosciences, Roosendaal, The Netherlands, http://www.amersham.com). Signals were visualized on x-ray film (Konica Minolta, Nieuwegein, The Netherlands, http://www.konicaminolta.com) and quantified. The intensities of the protein signals were quantified by using ImageJ software (NIH Image, Bethesda, MD). Measured intensities (pixel counts) of β-actin were used to normalize the intensities of the Ezh2 signals. Normalized intensities of Ezh2 samples were used to calculate the relative expression level of Ezh2. Statistical analysis of the results of three experiments was done by using Student's t test.
RT-PCR Analysis
RT-PCR and real-time quantitative PCR (qPCR) of Ezh2 expression was performed on mRNA collected from pooled undifferentiated NSCs, from pooled NSCs after differentiation, and from various differentiated neural cell types. For RT-PCR, the following primer pair for Ezh2 was used: forward 5′-GTC GGT GCA AAG CAC AAT GC-3′ and reverse 5′AGC TCC ACA CGT CAG ACA GAGG-3′. qPCR was performed by using the primer pair: forward 5′-TGG ACC ACA GTG TTA CCA GCA-3′ and reverse 5′-TGG GCG TTT AGG TGG TGT CT-3′. PCR amplification was performed by using SYBR Green in 96-well microtiter plates in an iCycler thermal cycler (Bio-Rad). The ΔCT method (by means of three reference genes: β-actin, HPRT1, and GAPDH), was used to perform the relative quantification among NSCs, different neural cell types, and OPC lines. To calculate the relative expression, Ezh2 expression was normalized by using a reference gene in each sample (2). The resulting expression values obtained (Fig. 2F) were indicated in relation to the expression in NSCs (= 1).
Immunocytochemistry
After culture periods of up to 5 days, NSC cultures were fixed with 4% paraformaldehyde and immunostained for markers of various neural cell types at various differentiation stages. The following antibodies were used: to identify neurons, anti-microtubule-associated protein-2 (AB5622, 1:500; Millipore-Chemicon, Amsterdam, The Netherlands, http://www.millipore.com); to identify astrocytes: anti-glial fibrillary acidic protein (MAB3402, 1:250; Millipore-Chemicon). The different cell stages of differentiation into oligodendrocytes were identified by anti-RIP (MAB1580, 1:250; Millipore-Chemicon), anti-platelet-derived growth factor receptor-α (1:250, Santa Cruz Biotechnology, Inc., Heidelberg, Germany, http://www.scbt.com), anti-NG2 (chondroitin sulfate proteoglycan, AB5320, 1:500; Millipore-Chemicon), anti-galactocerebroside (MAB342, 1:250; Millipore-Chemicon), and anti-myelin basic protein (MBP) (AB980, 1:500; Millipore-Chemicon). Undifferentiated NSCs were identified with anti-nestin. Expression of Ezh2 in NSCs and neural cell types was analyzed by using anti-Ezh2 antibody (1:250). Freshly differentiated astrocytes (astroblasts) were recognized by using anti-S100β (11420, 1:400; ICN Biomedicals, Inc., http://www.mpbio.com), whereas young neuroblasts were labeled with anti-polysialic acid (PSA)-NCAM (MAB5324, 1:300; Millipore-Chemicon,), or anti-doublecortin (DCX) (AB5910, 1:500; Millipore-Chemicon). Anti-KI67 (M7249, 1:20; DAKO) was used to demonstrate mitotic cells. Fluorescein isothiocyanate- or Cy3-conjugated secondary antibodies and Hoechst nuclear staining were used to identify the labeled and unlabeled cells. To stain and quantify dead cells we performed propidium iodide stainings by adding propidium iodide (5 μg/ml; Sigma-Aldrich) to the culture medium 5 hours before fixation.
Microscopy Analysis
Quantitative analysis of the cell differentiation pattern of NSCs without or after Ezh2 expression modulation was performed by using a Leica (SP2 AOBS) confocal laser scan microscope or a Zeiss (Axioskope 2) fluorescent microscope equipped with a Leica DFC300FX camera and the Leica microsystem LAS program. For differential cell type counting, based on cell type-specific immuno(double)staining, pictures at ×10 magnification were made of five randomly selected areas in the culture dish. Differential cell count analyses were done by using ImageJ software. In a similar manner, ImageJ software was used to measure the size of neurospheres in randomly taken pictures of neurosphere cultures. Statistical analysis of the results of three experiments was performed by using Student's t test.
Results
Expression of Ezh2 in Mouse Embryonic Neural Stem Cells
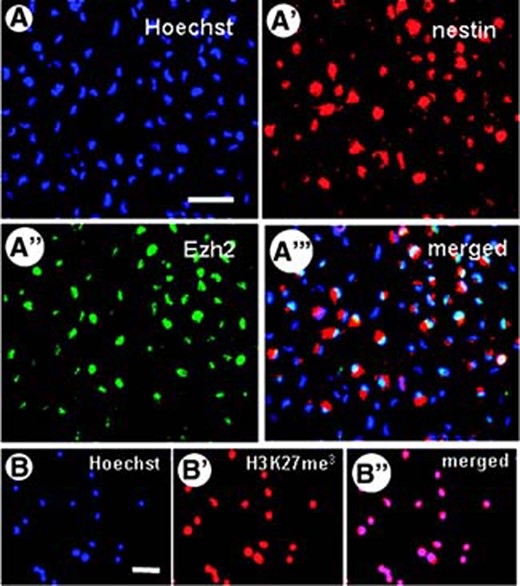
NSCs isolated from E14 mice were cultured in EGF- and bFGF-supplemented medium to maintain their undifferentiated state and to promote their proliferation, resulting in cell aggregates, the neurospheres. To detect Ezh2 expression in individual NSCs, we dissociated the neurospheres and fixated them to perform immunohistochemical analysis. All of the nestin-positive NSCs appear to express Ezh2 (Fig. 1A). Moreover, all NSCs, in accordance with the known activity of Ezh2, showed immunostaining for trimethylated lysine 27 histone H3 (H3K27me3) (Fig. 1B).
Expression of Ezh2 in mouse embryonic neural stem cells (NSCs). (A–A‴): After dissociation of the neurospheres, immunohistochemical staining shows that the individual nestin-positive NSCs (red fluorescence in cytoplasm) express Ezh2 (green fluorescence in nucleus); nuclear staining with Hoechst is indicated in blue. (B–B″): The presence of Ezh2 in all NSCs was associated with trimethylation of lysine 27 in histone 3, as demonstrated with an antibody directed against H3K27me3. Scale bars = 50 μm. Abbreviations: Ezh2, Enhancer of zeste homolog 2; H3K27m3, trimethylated H3K27.
Expression of Ezh2 During Differentiation of Mouse Embryonic Neural Stem Cells
Plating-dissociated mouse E14 NSCs in Neurobasal medium in poly-l-lysine/laminin-coated culture dishes resulted within 5 days in differentiation toward neurons, astrocytes, and oligodendrocytes. Double immunostaining for neural cell types and Ezh2 revealed the cell-specific expression of Ezh2: differentiation into astrocytes was accompanied by downregulation of the Ezh2 expression (Fig. 2A) and also during neuronal differentiation Ezh2 expression was weak (Fig. 2B). However, in NSCs that differentiated into an oligodendrocytic cell lineage, Ezh2 expression remained at an almost similar level as in undifferentiated NSCs (Fig. 2C). Western blot analysis and quantification on pure populations of primary undifferentiated NSCs, astrocytes, neurons, and (premyelinating) oligodendrocytes confirmed the findings observed in the mixed cell cultures of differentiated NSCs (Fig. 2D, 2D′). Moreover, analysis at the mRNA level, by using RT-PCR and qPCR, on pure suspensions of NSCs, primary astrocytes, neurons, and (premyelinating) oligodendrocytes revealed the same pattern of Ezh2 expression as demonstrated at the protein level (Fig. 2E, 2E′); the specific expression of Ezh2 during differentiation into oligodendrocyte cell lineage was further confirmed in three oligodendrocyte cell lines, that is, N19 (data not shown), N20.1, and Oli-neu cells (Fig. 2E, 2E′).
Expression of Ezh2 in mouse embryonic NSCs after differentiation. (A): NSCs differentiating into (GFAP-positive) astrocytes downregulate the expression of Ezh2, whereas (B) those differentiating into (MAP2-positive) neurons show a prominent reduction in Ezh2 expression. (C): However, NSCs differentiated into an oligodendrocyte cell lineage (RIP-positive) maintain about the same level of expression of Ezh2 as undifferentiated NSCs (insert in C′ shows the same cells only stained for Ezh2 [arrows]). (D): Western blot analysis and quantification (D′) on pure populations of primary undifferentiated NSCs, astrocytes, neurons, and (premyelinating) oligodendrocytes confirmed the findings observed in the mixed cell cultures of differentiated NSCs. (E): Reverse transcription (RT)-polymerase chain reaction (PCR) analysis of the expression of Ezh2 in pure suspensions of the following: 1, NSCs; 2, primary astrocytes; 3, neurons; 4, (premyelinating) oligodendrocytes; 5, Oli-neu cell line, undifferentiated, 6, Oli-neu cell line, differentiated, 7, N20.1 cell line, undifferentiated; and 8, N20.1 cell line, differentiated. (E′): Quantitative PCR (qPCR) analysis of the expression of Ezh2 in pure suspensions of primary undifferentiated NSCs, astrocytes, neurons, and (premyelinating) oligodendrocytes and undifferentiated and differentiated Oli-neu cells. Both RT-PCR and qPCR analyses revealed the same pattern for Ezh2 mRNA as demonstrated at the protein level in Western blots. Bar in (A–C): 50 μm. Abbreviations: Astro, astrocytes; D, differentiated; Ezh2, enhancer of zeste homolog 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFAP, glial fibrillary acidic protein; MAP2, microtubule-associated protein 2; NSC, neural stem cell; Neuro, neurons; Oligo, (premyelinating) oligodendrocytes; U, undifferentiated.
Overexpression of Ezh2 in Mouse Embryonic Neural Stem Cells
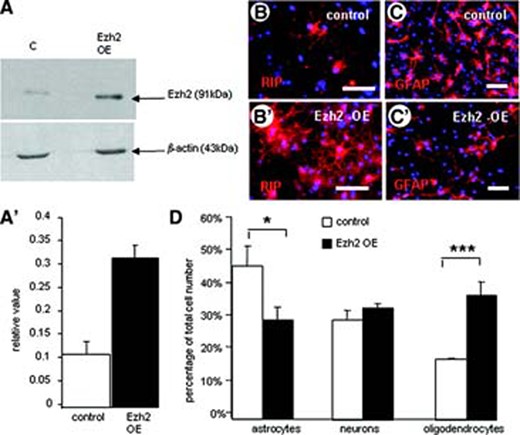
To investigate the involvement of Ezh2 in the differentiation process of mouse E14 NSCs, we have induced transient overexpression of Ezh2. An increase in intracellular Ezh2 levels was confirmed by using Western blot analysis (Fig. 3A, 3A′).
Overexpression of Ezh2 in mouse embryonic neural stem cells (NSCs). (A, A′): Western blot analysis shows the significant increase in Ezh2 expression due to Ezh2 gene transfection. (B, C): Overviews of immunohistochemical stainings for RIP (B, B′) and GFAP (C, C′) showing the effect of Ezh2 overexpression on the in vitro differentiation of NSCs in, respectively, oligodendrocytes and astrocytes compared with controls (B, C). Scale bars = 50 μm. (D): Quantification of the differentiation pattern of NSCs with Ezh2 overexpression into the three cell types expressed as a percentage of the total cell number (determined with Hoechst nuclear staining). Ezh2 overexpression leads to a significant reduction in the percentage of astrocytes and a significant increase in the percentage of oligodendrocytes (means of three independent experiments ± SE; ***, p < .001; *, p < .05; Student's t test). Abbreviations: Ezh2, enhancer of zeste homolog 2; GFAP, glial fibrillary acidic protein; OE, overexpression.
Plating Ezh2-overexpressing NSCs for differentiation resulted in a significant change in the differentiation pattern compared with eGFP-transfected control NSCs. Besides a slight increase in the percentage of neurons, Ezh2 overexpression in differentiating NSCs resulted in a reduction in astrocytes and a significant increase in the percentage of oligodendrocytes (Fig. 3B–3D).
Silencing of Ezh2 Expression in Mouse Embryonic Neural Stem Cells
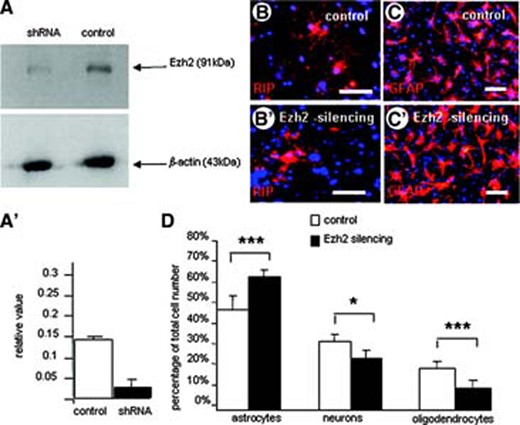
In addition, we have reduced the intracellular level of Ezh2 in mouse E14 NSCs by using transfection of shRNA (Fig. 4A, 4A′) and studied its effect on NSC proliferation and differentiation. Silencing of Ezh2 in undifferentiated NSCs appeared to reduce or slow down their proliferation, because the size of neurospheres 24 hours after plating was significantly smaller (data not shown). Differentiation of NSCs in which the Ezh2 level was reduced by silencing resulted in an inverse differentiation pattern compared to NSCs overexpressing Ezh2. Thus, silencing Ezh2 in differentiating NSCs significantly reduced the percentage of oligodendrocytes and promoted the generation of astrocytes, whereas the number of neurons was slightly decreased (Fig. 4B–4D).
Silencing of the Ezh2 expression in mouse embryonic neural stem cells (NSCs). (A, A′): Western blot analysis shows the significant decrease in Ezh2 expression in NSCs. (B, C): Overviews of immunohistochemical stainings for RIP (B, B′) and GFAP (C, C′) showing the effect of Ezh2 silencing on the in vitro differentiation of NSCs in, respectively, oligodendrocytes and astrocytes compared with controls (B, C). Scale bars = 50 μm. (D): Quantification of the differentiation pattern of NSCs with reduced Ezh2 expression into the three cell types expressed as percentage of the total cell number (determined with Hoechst nuclear staining). Ezh2 silencing leads to a significant reduction in the percentage of oligodendrocytes and a significant percentage in astrocyte differentiation (means of three independent experiments ± SE; ***, p < .001; *, p < .05; Student's t test). Abbreviations: Ezh2, enhancer of zeste homolog 2; GFAP, glial fibrillary acidic protein; shRNA, short hairpin silencing RNA.
Involvement of Ezh2 in Oligodendrocyte Generation
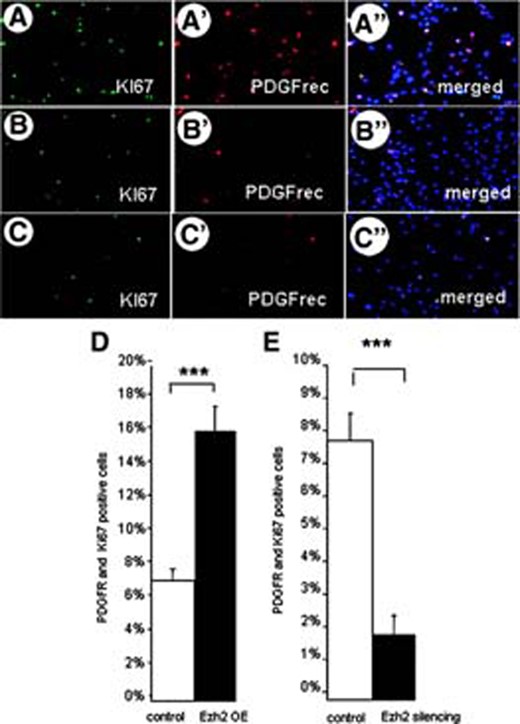
The increase in the number of oligodendrocytes differentiating from Ezh2-overexpressing NSCs may be due to selective survival, selective stimulation of OPC proliferation, and/or a direct oligodendrocyte differentiation stimulating effect. As far as selective survival is concerned, no differences in cell death were observed between differentiating control NSCs and Ezh2-overexpressing or silenced NSCs (data not shown). To determine whether the increase in the number of oligodendrocytes after differentiation of Ezh2-overexpressing NSCs could be ascribed to a selective Ezh2-mediated stimulation of proliferation of freshly formed OPCs, we examined the proliferative activity of the PDGFrec-positive OPCs with the use of the mitosis marker KI67 (Fig. 5A–5C). Indeed, Ezh2 overexpression significantly doubled the proliferation of OPCs (Fig. 5D); Ezh2 silencing, in contrast, significantly reduced OPC proliferation (Fig. 5E).
Oligodendrocyte precursor cell (OPC) proliferation and Ezh2 expression. The number of proliferating OPCs is determined by using double immunostaining for KI67 and PDGFrec α after inducing Ezh2 overexpression (A–A″) in control conditions (B–B″) and after Ezh2 silencing (C–C″). Quantification in (D) showed that overexpression of Ezh2 in freshly differentiated OPCs stimulates their proliferation, whereas silencing of Ezh2 expression (E) results in a decrease of proliferating OPCs (means of three independent experiments ± SE; ***, p < .001, Student's t test). Abbreviations: Ezh2, enhancer of zeste homolog 2; OE, overexpression; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor.
Ezh2 Expression During Oligodendrocyte Differentiation of Mouse Embryonic Neural Stem Cells
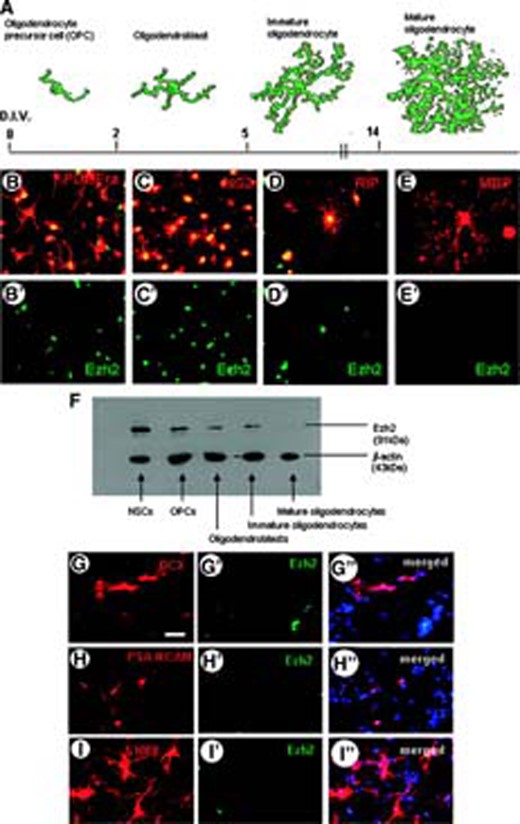
Apart from a role for Ezh2 in regulating OPC proliferation, its selective expression in oligodendrocytic cell lineage may still point to an involvement in oligodendrocyte differentiation and maturation. We examined the expression of Ezh2 at subsequent stages of oligodendrocyte differentiation and maturation (Fig. 6A) in more detail by performing double immunolabeling for Ezh2 and marker proteins of specific oligodendrocyte differentiation stages taken at different time points during oligodendrocyte differentiation/maturation. Besides the early OPC stage we subsequently distinguished the oligodendroblast, the immature and the mature oligodendrocyte stage. Cell proliferation could only be detected in the short OPC stage. Ezh2 expression was evident from the OPC stage up to the immature oligodendrocyte stage, but was downregulated in the mature (myelinating) oligodendrocyte characterized by the start of MBP production (Fig. 6B–6E). Western blot analysis at different stages of oligodendrocyte differentiation confirmed the immunocytochemical findings (Fig. 6F). The specific expression of Ezh2 in differentiating oligodendrocytes may merely reflect a difference in the efficiency and speed to differentiate in vitro in comparison with differentiating astrocytes and neurons. Ezh2 expression in undifferentiated NSCs may just gradually disappear during differentiation and, consequently, in cell types with an extended differentiation pathway (oligodendrocytes) it can be detected longer than in cell types (astrocytes or neurons) with a short differentiation pathway. Therefore, we have tried to detect Ezh2 at the earliest stages of astrocyte and neuron differentiation by using specific early markers, such as S100β (for freshly formed astrocytes) and DCX and PSA-NCAM for young neuroblasts. In both cases the expression of Ezh2 was not detectable (Fig. 6G–6I).
Expression of Ezh2 during various stages of in vitro differentiation of mouse embryonic NSCs. (A): Schematic drawings of the stages of oligodendrocyte differentiation (appearing after indicated days in vitro) that are shown in (B–E), immunostained (red fluorescent) for PDGFR-α, NG2, RIP, and MBP, respectively. Double staining of these stages for Ezh2 (green fluorescent, singular Ezh2 staining in B′–E′) shows that Ezh2 is expressed during all stages of oligodendrocyte differentiation but not in the mature oligodendrocytes. (F): Western blot analysis of Ezh2 expression in the various oligodendrocyte stages confirms the immunohistochemical findings. (G–I): Double immunostaining for Ezh2 and DCX (G–G″), PSA-NCAM (H–H″) and S100β (I) shows that, respectively, early neuroblasts (G–H) and freshly formed astroblasts (I–I″) do not express Ezh2 (Hoechst staining is indicated by blue). Scale bar in G for G–I″ = 50 μm. Abbreviations: DCX, doublecortin; D.I.V., days in vitro; Ezh2, enhancer of zeste homolog 2; MBP, myelin basic protein; NSC, neural stem cell; OPC, oligodendrocyte precursor cell; PDGFRa, platelet-derived growth factor receptor-α; PSA, polysialic acid.
Discussion
Our studies show that NSCs derived from E14 mouse brain express the PcG protein Ezh2. Whereas Ezh2 expression in NSCs is downregulated on differentiation into neurons and particularly in astrocytes, surprisingly, its expression remains at a high level in freshly differentiated proliferative OPCs and at subsequent stages of oligodendrocyte differentiation and only disappears in mature oligodendrocytes. Interference with Ezh2 expression in NSCs during proliferation and differentiation reveals the involvement of Ezh2 in the regulation of NSC and OPC proliferation and presumably in oligodendrocyte differentiation and maturation.
A number of studies have shown that PcG proteins are important for the maintenance and self-renewal of embryonic stem cells and various adult stem cells, such as hematopoietic stem cells, muscle precursor cells, and NSCs [1–3, 6]. In general, in line with its function described for the development of Drosophila, the PcG repressor complexes repress the transcription of gene programs encoding differentiation into a specific cell lineage and retain stem cells in a proliferative stage. It has been shown, for instance, that the proliferation of hematopoietic stem cells is regulated by a balance between the PRC2 and PRC1 components Eed and Bmi1 [10, 11], whereas overexpression of Ezh2 preserves the long-term repopulating potential of these cells over serial transplantation, preventing aging and exhaustion [16]. The PcG protein Bmi1, a component of PRC1, has been shown to be involved in the maintenance and proliferation regulation of adult NSCs [10–14]; besides severe proliferation defects during hematopoiesis, Bmi1-deficient mice exhibit aberrant transformations coupled with acute neurological disorders [15]. In a recent laser capture microdissection and microarray analysis (Gurok et al., 2007), high Ezh2 expression was detected selectively in proliferating adult NSCs in the hippocampus [17]. Our present findings on the expression of Ezh2 in undifferentiated proliferating NSCs in vitro and its dowregulation on differentiation into astrocytes and neurons, seem to be in accordance with a potential role in the regulation in NSC self-renewal and maintenance of stemness.
Unexpectedly, differentiation of NSCs into oligodendrocytes did not result in the downregulation of Ezh2 expression. The expression of Ezh2 in OPCs may reflect the multipotency ascribed to rat OPCs by Kondo and Raff [21, 22]. They showed that, with proper extracellular stimuli, cultured rat OPCs could be dedifferentiated back toward type 2 astrocytes, which subsequently could be stimulated to proliferate and to behave like NSCs, producing neurons, astrocytes, and oligodendrocytes. To further establish a potential role of Ezh2 in OPC multipotency, it may be relevant to correlate Ezh2 down-regulation in rat OPCs with loss of multipotency (the Kondo-Raff protocol to induce OPC multipotency appeared to be ineffective in our cultured mouse OPCs). Ezh2 overexpression and silencing in differentiating NSCs resulted in an increase and a reduction in the number of oligodendrocytes, respectively. Because no indications were found for differences in selective cell death, the increase in oligodendrocytes differentiating from overexpressing Ezh2 must be ascribed to selective stimulation of OPC proliferation or/and a direct oligodendrocyte differentiation-stimulating effect. The observed increase in proliferation of OPCs by Ezh2 seems to be in line with the role of Ezh2 in self-renewal and proliferation described in other stem cell types, such as hematopoietic stem cells. Whether the proliferation of OPCs is regulated by a balance between the PRC2 and PRC1 components as in hematopoietic stem cells [11] still has to be elucidated, but so far we were unable to detect Bmi1 expression in proliferating OPCs (unpublished data).
Besides high expression in OPCs, Ezh2 remained upregulated during the subsequent stages of oligodendrocyte differentiation, that is, in oligodendroblasts up to immature (premyelinating) oligodendrocytes arising in culture around day 14. Ezh2 expression is only downregulated at the time the oligodendrocyte reaches its mature myelination stage. The possibly different role of Ezh2 in proliferating OPCs and in oligodendrocyte differentiation may point to different target genes or to differences in the composition and activity of the PRC complexes involved. The process of differentiation into an oligodendrocyte cell lineage is prominently associated with gene derepression, that is, the silencing of oligodendrogenic gene repressors [23]. The presence of an Ezh2 PRC2 complex in differentiating oligodendrocytes is in line with the findings that this complex has been linked to recruitment of histone deacetylase activity [24] and that histone deacetylase activity is essential for oligodendrocyte differentiation and maturation [25]. PRC2 initiates transcriptional repression and in that process the Ezh2-mediated H3K27 trimethylation leads to the recruitment of PRC1, which is thought to maintain repressive conditions. Indeed, Ezh2-mediated H3K27 trimethylation could be demonstrated in NSCs and OPCs, but the recruitment of PRC1, subsequent continuous gene transcription repression, and the genes targeted have yet to be demonstrated. Three studies [6–8] have indicated that the most prominent target gene repressed via the PRC2 complex in embryonic stem cells is Olig2. Because Olig2 is a gene crucially involved in oligodendrocyte specification [26–28], it seems unlikely that this gene is repressed via Ezh2 and subsequent PRC1 recruitment in the oligodendrocytic cell lineage generated in NSCs. It has been demonstrated that the actual recruitment of PRC1 by the PRC2 complex depends on the composition of the PRC2 complex and the properties of the components involved: a number of Ezh2 PRC2 complexes have been identified, which can contain, for instance, at least four different Eed isoforms [29, 30], differently interacting with Ezh2 and leading to different recruitment strategies of PRC complexes (besides PRC1, also PRC3 and PRC4). The composition of the PRCs, their histone substrate specificity, and thus the action mediated via Ezh2 are highly dynamic and distinct at different stages in cell differentiation [9, 31]. PRCs may differentially regulate the expression of the different transcription factors that determine the transition from one stage of differentiation into the next during OPC maturation [32].
In conclusion, our data show for the first time the involvement of the Polycomb group protein Ezh2 in proliferation and differentiation of NSC-derived oligodendrocytes. The challenge for further research is to unravel the complex molecular mechanisms underlying the regulation by Ezh2 and other PcG proteins during the differentiation and maturation of oligodendrocytes.
Acknowledgements
We thank Professor G. de Haan (Department of Cell Biology, Stem Cell Biology, University Medical Centre Groningen, University of Groningen, Groningen, The Netherlands) for helpful discussions, and Dr. M.J. Leroy (University Rene Descartes, Paris, France) and Dr. J. Trotter (Johannes Gutenberg University of Mainz, Mainz, Germany) for providing us the oligodendrocyte cell lines. We gratefully acknowledge the technical assistance of E. Wesselink. This research was supported by a grant of the Dutch Foundation MS Research (MS 04-554MS).
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
Author notes
Author contributions: F.S.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; R.R. and N.B.: collection and assembly of data, data analysis and interpretation; V.B.: conception and design, data analysis and interpretation; E.B.: conception and design, data analysis and interpretation, manuscript writing; S.C.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.

![Expression of Ezh2 in mouse embryonic NSCs after differentiation. (A): NSCs differentiating into (GFAP-positive) astrocytes downregulate the expression of Ezh2, whereas (B) those differentiating into (MAP2-positive) neurons show a prominent reduction in Ezh2 expression. (C): However, NSCs differentiated into an oligodendrocyte cell lineage (RIP-positive) maintain about the same level of expression of Ezh2 as undifferentiated NSCs (insert in C′ shows the same cells only stained for Ezh2 [arrows]). (D): Western blot analysis and quantification (D′) on pure populations of primary undifferentiated NSCs, astrocytes, neurons, and (premyelinating) oligodendrocytes confirmed the findings observed in the mixed cell cultures of differentiated NSCs. (E): Reverse transcription (RT)-polymerase chain reaction (PCR) analysis of the expression of Ezh2 in pure suspensions of the following: 1, NSCs; 2, primary astrocytes; 3, neurons; 4, (premyelinating) oligodendrocytes; 5, Oli-neu cell line, undifferentiated, 6, Oli-neu cell line, differentiated, 7, N20.1 cell line, undifferentiated; and 8, N20.1 cell line, differentiated. (E′): Quantitative PCR (qPCR) analysis of the expression of Ezh2 in pure suspensions of primary undifferentiated NSCs, astrocytes, neurons, and (premyelinating) oligodendrocytes and undifferentiated and differentiated Oli-neu cells. Both RT-PCR and qPCR analyses revealed the same pattern for Ezh2 mRNA as demonstrated at the protein level in Western blots. Bar in (A–C): 50 μm. Abbreviations: Astro, astrocytes; D, differentiated; Ezh2, enhancer of zeste homolog 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFAP, glial fibrillary acidic protein; MAP2, microtubule-associated protein 2; NSC, neural stem cell; Neuro, neurons; Oligo, (premyelinating) oligodendrocytes; U, undifferentiated.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/stmcls/26/11/10.1634_stemcells.2008-0121/3/m_stmcls_26_11_2875_nfig002.jpeg?Expires=1716964658&Signature=qyg4TXxmdsAB~eYsgzSlSBmK-Vfb-TWurJgmJxcVOak1LxSQBaZ2HWBkveN8GyuyPW4oFm6gIGw8p9v1VLdR~EgvWPVyQr8aOQa69d74kMX9omjifA58yXhHtznYfQHTTbmxj0hfxSG-qwvxneZTwm7-9WxkVrr5SWxH9IzDuXpq5D5Ghx1HC1su26R749Yewj~ozSEaVoGxE21iDtf2uIhooEDFQFJHGe6fOmAbf1Fr79CLwMAeNRAorSMr9B1iCV1yE1R~L3fzWrZ7roIkh2i78croG6Fs9ZxGxjKIQVjgnvR31CgJRYIWQXTkyshMEqwUdq0N4C5CssSeXENRCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)