-
PDF
- Split View
-
Views
-
Cite
Cite
G. Frank Gerberick, John A. Troutman, Leslie M. Foertsch, Jeffrey D. Vassallo, Mike Quijano, Roy L. M. Dobson, Carsten Goebel, Jean-Pierre Lepoittevin, Investigation of Peptide Reactivity of Pro-hapten Skin Sensitizers Using a Peroxidase-Peroxide Oxidation System, Toxicological Sciences, Volume 112, Issue 1, November 2009, Pages 164–174, https://doi.org/10.1093/toxsci/kfp192
Close - Share Icon Share
Abstract
Skin protein reactivity is a well established key step in the development of skin sensitization. Understanding the relationship between a chemical's ability to react with or modify skin protein and skin sensitization has led to the development of the Direct Peptide Reactivity Assay (DPRA) in our laboratory. A current limitation of the DPRA is that it cannot readily measure the reactivity of pro-hapten chemical sensitizers. Pro-haptens are chemical sensitizers that are not directly reactive and must be bioactivated in vivo to form an electrophilic intermediate(s). Results from this work demonstrate the utility of using horseradish peroxidase and hydrogen peroxide (HRP/P) for assessing the skin sensitization potential of pro-haptens. In comparison with “direct” reactivity assessments without HRP/P, statistically significant increases in peptide depletion for all pro-haptens examined were observed following coincubation with HRP/P. Conversely, the percent peptide depletion for all pre-haptens was equally high (> 40% depletion) with and without HRP/P demonstrating an auto-oxidation pathway. In contrast, peptide depletion for all nonsensitizing chemicals examined was low with and without HRP/P. The optimal HRP/P concentrations, incubation time and optimal peptide:chemical ratio were determined using a sensitive and selective high-performance liquid chromatography tandem mass spectrometry detection method. Dithiothreitol was incorporated to reverse the dimerization of the thiol-containing cysteine peptide nucleophile. This preliminary work shows the potential to incorporate an enzyme-mediated activation step for pro-haptens into an in chemico skin sensitization assay that results in the detection of all types of sensitizers.
The correlation of skin protein reactivity and skin sensitization is well established (Dupuis and Benezra, 1982; Gerberick et al., 2008; Landsteiner and Jacobs, 1936; Lepoittevin et al., 1998). Although a variety of mechanisms contribute to protein reactivity, it is generally recognized that this process involves the reaction of a small molecule, having electrophilic properties, with a nucleophilic amino acid on a protein. The majority of chemical allergens (or their metabolites) have electrophilic properties and are able to react with various nucleophiles to form covalent bonds. Lysine and cysteine are those most often cited, but other amino acids containing nucleophilic heteroatoms (e.g., histidine, methionine) can also react with electrophiles (Ahlfors et al., 2003; Alvarez-Sanchez et al., 2003; Dupuis and Benezra, 1982; Lepoittevin et al., 1998; Meschkat et al., 2001). Thus, an allergen must form a stable association with proteins, in order that an immunogenic complex is created for recognition by the immune system (Gerberick et al., 2008). However, to date the exact nature of relevant covalent modifications in vivo has yet to be determined empirically for specific allergens (Divkovic et al., 2005).
Investigators have been interested in pursuing whether or not measuring a chemical's reactivity could be used to develop an in chemico quantitative peptide-based reactivity assay that would have utility for screening a chemical's skin sensitization potential as defined in the Local Lymph Node Assay (LLNA) (Aleksic et al., 2009; Aptula et al., 2006; Gerberick et al., 2004, 2007; Kato et al., 2003; Natsch and Gfeller, 2008; Natsch et al., 2007; Roberts and Natsch, 2009; Schultz et al., 2005). This is of particular importance in view of the forthcoming European Union ban on in vivo testing of cosmetic and toiletry ingredients following the publication of the 7th Amendment to the Cosmetic Directive (European Union 7th Amendment to Cosmetic Directive).
We (Gerberick et al., 2004, 2007) have developed a Direct Peptide Reactivity Assay (DPRA) for screening the skin sensitization potential of chemicals by measuring peptide depletion following incubation with allergens and nonallergens. The heptapeptides used in the DPRA, which have either a cysteine or a lysine, were incubated at a peptide to chemical ratio of 1:10 and 1:50, respectively. Following a 24-h reaction period of test chemical with the two synthetic peptides, the samples were analyzed by high-performance liquid chromatography (HPLC) using ultraviolet (UV) detection to monitor the depletion of peptide following reaction. Initial results using 82 chemicals representing allergens of different potencies (weak to extreme) and nonsensitizers indicated a strong correlation between allergen potency and depletion of the peptides (Gerberick et al., 2004, 2007). The peptide reactivity data were compared with existing Local Lymph Node Assay (LLNA) EC3 (mathematically Estimated Concentration of chemical required to induce a threefold stimulation index) potency data (Kimber et al., 2003) using recursive partitioning methodology to build a classification tree model that allowed a ranking of reactivity as minimal, low, moderate and high. Classifying minimal reactivity as nonsensitizers and low, moderate and high reactivity as sensitizers, it was determined that a model based on cysteine (1:10) and lysine (1:50) gave a prediction accuracy of 89%.
As a test chemical may be a pre-hapten or a pro-hapten (Lepoittevin, 2006; Smith and Hotchkiss, 2001), it is critical to incorporate methods that allow for the formation of a protein reactive hapten through either spontaneous air-oxidation (pre-hapten) or enzyme-mediated activation (pro-hapten) which simulate biotransformation in the skin. The DPRA described above is designed specifically for haptens that have ability within their structure to react directly with peptides or proteins containing nucleophilic amino acids such as cysteine or lysine. In some instances pre-haptens can be detected in the DPRA (Gerberick et al., 2007) but pro-haptens would not unless an enzyme system was added to the assay to convert the pro-haptens to reactive molecules.
We evaluated a pragmatic approach for the enzymatic activation of pro-haptens that involves the use of a horseradish peroxidase and hydrogen peroxide (HRP/P) oxidation system. As peroxidase-dependent oxidation reactions play important roles in the activation of xenobiotics (Eling et al., 1990; Hofstra and Uetrecht, 1993), the HRP/P system is considered to provide biologically relevant activation. Specifically, a quantitative enzyme-based in chemico method using HRP/P has been developed for measuring peptide reactivity of chemical sensitizers considered to be pro-haptens. Sample analysis is performed by using a highly sensitive and selective high-performance liquid chromatography and tandem mass spectrometry (HPLC/MS/MS) detection method that is rapid and amenable to high-throughput screening. In this report, we present the successful use of this system to examine peptide depletion by pro-haptens as well as the optimization of the experimental conditions necessary for the assessment of pro-haptens of different sensitization potencies. With further refinements that may include the use of different nucleophiles and/or enzyme systems and with subsequent testing using a much larger set of sensitizing and nonsensitizing chemicals, perhaps a more holistic and comprehensive in vitro assessment for predicting the sensitization potential of all possible chemical allergens (i.e., haptens, pre-haptens, and pro-haptens) will emerge. The method described here has the potential to form the basis of such a model.
MATERIALS AND METHODS
Test chemicals and reagents.
The following test chemicals with accompanying purity and CAS numbers were purchased from Aldrich Chemical Company (Milwaukee, WI): 2-aminophenol, 99% [95-55-6], 1-butanol, 99% [71-36-3], cinnamyl alcohol, 98% [104-54-1], eugenol, 99% [97-53-0], isoeugenol, 99% [97-54-1], 2-methoxy-4-methylphenol, 99% [93-51-6], 3-methylcatechol, 98% [488-17-5], 1-naphthol, 99% [90-15-3], and 1,4-phenylenediamine, 99% [106-50-3]. The following test chemicals with accompanying purity and CAS numbers were purchased from Sigma Chemical Company (St Louis, MO): aniline, 99.5% [62-53-3], hydroquinone, and 99% [123-31-9]. The following test chemicals with accompanying purity and CAS numbers were purchased from Fluka Chemical Company (Milwaukee, WI): 4-amino-m-cresol, 98% [2835-99-6], geraniol, 96% [106-24-1], and (+/−) lactic acid, 90% [598-82-3]. Isopropanol [67-63-0] was purchased from EM Science (Gibbstown, NJ). Desferroxamine (catalog number D9533), horseradish peroxidase (catalog number P6140), hydrogen peroxide (catalog number 216763), and D,L-dithiothreitol (DTT) (catalog number D5545-16) were purchased from Aldrich Chemical Company (Milwaukee, WI). The cysteine peptide (Ac-RFAACAA-COOH, catalog number 05-02-23-01-PRG) was prepared and purified by the SynPep Corporation (Dublin, CA). The internal standard (leucine enkephalin, catalog number L9133) was purchased from Sigma-Aldrich Chemical Company (St Louis, MO).
Cysteine peptide incubation conditions.
Reactivity of test chemicals to cysteine-based synthetic peptide (20μM) was determined in triplicate reactions containing 10μM desferroxamine at test article concentrations of 200μM in 0.1M potassium phosphate buffer, pH 7.4. The final reaction volume was 0.3 ml. Unless otherwise indicated, enzyme-mediated reactivity was determined in samples containing HRP/P at a final concentration of 3 U/ml and 100μM, respectively. Reactivity in the absence of enzyme (direct reactivity) was assessed in test chemical incubations devoid of HRP/P. Incubations with cysteine peptide and no test chemical, in the absence and presence of HRP/P, served as zero-depletion reference controls for comparisons to direct and enzyme-mediated test chemical reactivity determinations, respectively. Sample reactions were initiated by adding 3 μl of a 20mM test chemical stock solution prepared in 100% methanol. Final organic content in each reaction was ≤ 1%. Unless otherwise indicated, samples were incubated under ambient lab conditions for 24 h.
Sample processing and analysis by HPLC/MS/MS.
Following the incubation period, samples were diluted with 0.3 ml of internal standard solution (3 μg/ml leucine enkephalin in 95/5 acetonitrile/water) and 10 μl of each sample was diluted with 390 μl of 2/98 acetonitrile/water. A 10-μl aliquot of a 16mM DTT solution (prepared in water) was then added and samples were heated in a 40°C oven for 30 min prior to analysis.
Analysis was primarily designed to selectively monitor two analytes: the cysteine peptide (monomer) and leucine enkephalin (internal standard). The HPLC/MS/MS methodology employed a CTC HTS-PAL autosampler (Leap Technologies, Carrboro, NC) for sample introduction. Chromatographic separation and detection was carried out using a 10ADvp pumping system (Shimadzu, Colombia, MD), interfaced to a Sciex API 3000 triple quadrupole mass spectrometer (Applies Biosystems, Foster City, CA), equipped with TurboIonSpray and operated in positive electrospray ionization. To achieve high selectivity and sensitivity, selected reaction monitoring (SRM) schemes were optimized for detection of the protonated (MH+) forms of the cysteine peptide (m/z 751–120) and leucine enkephalin (m/z 556–120). Note that the thiol-dimer form of the cysteine peptide was chromatographically detectable and monitored in the SRM channel of the targeted monomer (due to its [M′ + 2H]2+ form).
Injection volumes of 20 μl were made onto a YMC Pro C18 (2.1 × 20 mm, 3 μm) analytical column (Waters Corporation, Milford, MA). The mobile phase consisted of 2mM ammonium acetate in water, pH ∼ 8 (A), and 99/1 methanol/water, 2mM ammonium acetate, pH ∼8 (B). Separation was achieved using a 1.5-min linear gradient to 100% B from initial conditions of 95/5 A/B, with a total analysis time of 5 min per sample.
For typical batch analyses, involving multiple test compounds, the triplicate direct and enzyme-mediated reactivity sample sets were analyzed in alternating fashion and each of the six (total) sample sets was bracketed by a pair of the respective zero-depletion control samples. This allowed more-precise comparisons of reactivity between direct and enzyme-mediated conditions for each test compound. Also, by using the mean of the two bracketing control sample results (i.e., analyzed in closest temporal proximity to the test samples) optimal accuracy in depletion measurement was achieved.
Data reduction for peptide depletion measurements.
SRM peak areas and area ratios (cysteine peptide-to-internal standard) were determined for each sample, using the Sciex Analyst 1.4 quantitation software package (Applied Biosystems, Foster City, CA). Peptide depletion in samples with test chemical was calculated by comparing the peak area ratio in samples containing test chemical to the average of the peak area ratio that was calculated from the corresponding pair of bracketing control samples. Peptide depletion values for individual replicates were then used to calculate a mean and standard deviation for both the direct (no HRP/P) and enzyme-mediated (with HRP/P) reactivity determinations.
Statistical analysis.
Statistical analysis was conducted on the logit-transformed peptide depletion values. A binary logit transformation (log(p/(1 − p))) was used in order to allow for the use of parametric techniques, which assume normal distribution of the data. The transformed data were analyzed via ANOVA using sensitization category, compound within category, experiment, and whether enzyme was used (yes/no) as factors in the model. ANOVA F-tests were conducted in order to determine whether significant differences exist in the response based on whether HRP/P was coincubated (yes/no) with each test chemical. Statistical significance was determined at the 0.05 level. SAS software (SAS/STAT software, Version 9.2 of the SAS System for Windows; Copyright ©2008 SAS Institute Inc., Cary, NC) was used to conduct the analysis.
RESULTS
Test Chemicals: Pre-haptens and Pro-haptens
The skin sensitizers used in this study were chosen because they have been reported to be pre- or pro-haptens that are nonelectrophilic and require enzymatic or nonenzymatic activation to form protein reactive intermediates (Bertrand et al., 1997; Smith and Hotchkiss, 2001; Smith-Pease et al., 2003). The pre-haptens (hydroquinone, 4-amino-m-cresol, 3-methylcatechol, isoeugenol, and 1,4-phenylenediamine) and pro-haptens (geraniol, cinnamic alcohol, aniline, 2-aminophenol, 1-naphthol, eugenol, and 2-methoxy-4-methylphenol) represent different chemistries and include aliphatic alcohols and various hydroxylated or aminated aromatic derivatives likely to represent different reaction mechanisms for modifying proteins (i.e., Michael acceptor, SN2 electrophiles, SNAr electrophiles, Schiff base formers, acyl transfer electrophiles). In addition, the pre- and pro-haptens represent weak, moderate, and strong/extreme skin sensitizers based on available LLNA data (Table 1). The nonsensitizing molecules that were included as negative controls were 1-butanol, (+/−) lactic acid, and isopropanol.
Test Chemicals Used in this Study and LLNA Potency Data
| Chemical name | CAS # | LLNA EC3 (%)a |
| Nonsensitizers | ||
| 1-Butanol | 71-36-3 | NCb |
| (+/−) Lactic acid | 598-82-3 | NC |
| Isopropanol | 67-63-0 | NC |
| Weak sensitizers | ||
| Aniline | 62-53-3 | 89 |
| Geraniol | 106-24-1 | 26 |
| Cinnamyl alcohol | 104-54-1 | 21 |
| Eugenol | 97-53-0 | 13 |
| Moderate sensitizers | ||
| 2-Methoxy-4-methylphenol | 93-51-6 | 5.8 |
| 4-Amino-m-cresol | 2835-99-6 | 1.5 |
| 1-Naphthol | 90-15-3 | 1.3 |
| Isoeugenol | 97-54-1 | 1.2 |
| Strong/extreme sensitizers | ||
| 2-Aminophenol | 95-55-6 | 0.40 |
| 1,4-Phenylenediamine | 106-50-3 | 0.16 |
| Hydroquinone | 123-31-9 | 0.11 |
| 3-Methylcatechol | 488-17-5 | 0.020 |
| Chemical name | CAS # | LLNA EC3 (%)a |
| Nonsensitizers | ||
| 1-Butanol | 71-36-3 | NCb |
| (+/−) Lactic acid | 598-82-3 | NC |
| Isopropanol | 67-63-0 | NC |
| Weak sensitizers | ||
| Aniline | 62-53-3 | 89 |
| Geraniol | 106-24-1 | 26 |
| Cinnamyl alcohol | 104-54-1 | 21 |
| Eugenol | 97-53-0 | 13 |
| Moderate sensitizers | ||
| 2-Methoxy-4-methylphenol | 93-51-6 | 5.8 |
| 4-Amino-m-cresol | 2835-99-6 | 1.5 |
| 1-Naphthol | 90-15-3 | 1.3 |
| Isoeugenol | 97-54-1 | 1.2 |
| Strong/extreme sensitizers | ||
| 2-Aminophenol | 95-55-6 | 0.40 |
| 1,4-Phenylenediamine | 106-50-3 | 0.16 |
| Hydroquinone | 123-31-9 | 0.11 |
| 3-Methylcatechol | 488-17-5 | 0.020 |
LLNA potency data were from Gerberick et al. (2005), except for 4-amino-m-cresol (SCCP [EU Scientific Committee on Consumer Products], 2005) and 3-methylcatechol (unpublished data).
NC = not calculated.
Test Chemicals Used in this Study and LLNA Potency Data
| Chemical name | CAS # | LLNA EC3 (%)a |
| Nonsensitizers | ||
| 1-Butanol | 71-36-3 | NCb |
| (+/−) Lactic acid | 598-82-3 | NC |
| Isopropanol | 67-63-0 | NC |
| Weak sensitizers | ||
| Aniline | 62-53-3 | 89 |
| Geraniol | 106-24-1 | 26 |
| Cinnamyl alcohol | 104-54-1 | 21 |
| Eugenol | 97-53-0 | 13 |
| Moderate sensitizers | ||
| 2-Methoxy-4-methylphenol | 93-51-6 | 5.8 |
| 4-Amino-m-cresol | 2835-99-6 | 1.5 |
| 1-Naphthol | 90-15-3 | 1.3 |
| Isoeugenol | 97-54-1 | 1.2 |
| Strong/extreme sensitizers | ||
| 2-Aminophenol | 95-55-6 | 0.40 |
| 1,4-Phenylenediamine | 106-50-3 | 0.16 |
| Hydroquinone | 123-31-9 | 0.11 |
| 3-Methylcatechol | 488-17-5 | 0.020 |
| Chemical name | CAS # | LLNA EC3 (%)a |
| Nonsensitizers | ||
| 1-Butanol | 71-36-3 | NCb |
| (+/−) Lactic acid | 598-82-3 | NC |
| Isopropanol | 67-63-0 | NC |
| Weak sensitizers | ||
| Aniline | 62-53-3 | 89 |
| Geraniol | 106-24-1 | 26 |
| Cinnamyl alcohol | 104-54-1 | 21 |
| Eugenol | 97-53-0 | 13 |
| Moderate sensitizers | ||
| 2-Methoxy-4-methylphenol | 93-51-6 | 5.8 |
| 4-Amino-m-cresol | 2835-99-6 | 1.5 |
| 1-Naphthol | 90-15-3 | 1.3 |
| Isoeugenol | 97-54-1 | 1.2 |
| Strong/extreme sensitizers | ||
| 2-Aminophenol | 95-55-6 | 0.40 |
| 1,4-Phenylenediamine | 106-50-3 | 0.16 |
| Hydroquinone | 123-31-9 | 0.11 |
| 3-Methylcatechol | 488-17-5 | 0.020 |
LLNA potency data were from Gerberick et al. (2005), except for 4-amino-m-cresol (SCCP [EU Scientific Committee on Consumer Products], 2005) and 3-methylcatechol (unpublished data).
NC = not calculated.
Depletion of Cysteine Peptide in the Presence and Absence of HRP/P and Utility of Dithiothreitol
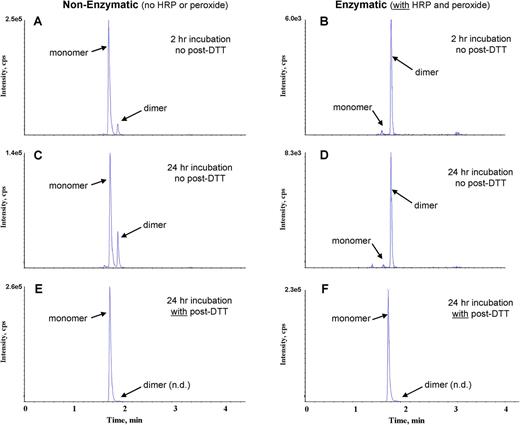
In the absence of any test chemicals, a preliminary study was conducted to monitor the selective loss of cysteine peptide monomer by HPLC/MS/MS, following incubation with and without HRP (6.7 U/ml) and hydrogen peroxide (0.9mM). In comparison with incubations without HRP/P, coincubation of cysteine peptide with HRP/P for 2 or 24 h resulted in virtually the complete loss of the peptide monomer (retention time [RT] = 1.74 min) and an increase in the dimeric form of the peptide (RT = 1.83 min) (Figs. 1A–D). In incubations without HRP/P, depletion of peptide monomer and formation of dimer did occur (Figs. 1A and 1C) but the observed effect was much less extensive than was observed in samples containing HRP/P (Figs. 1B and 1D). Subsequent treatment of the 24-h samples with DTT (post-DTT) resulted in the reduction of the disulfide bonds in thiol-dimers with conversion of the peptide back to the monomeric form (Figs. 1E, 1F).
Representative HPLC/MS/MS chromatograms from 2- or 24-h incubations containing cysteine peptide (without any test chemical) in the absence of HRP/P (A, C, E) and in the presence of HRP/P (B, D, F). Subsequent to the 2- or 24-h incubation period, samples were either processed without postincubation DTT (post-DTT) treatment (A, B, C, D) or were treated with post-DTT (E, F) and analyzed. n.d. = not detected.
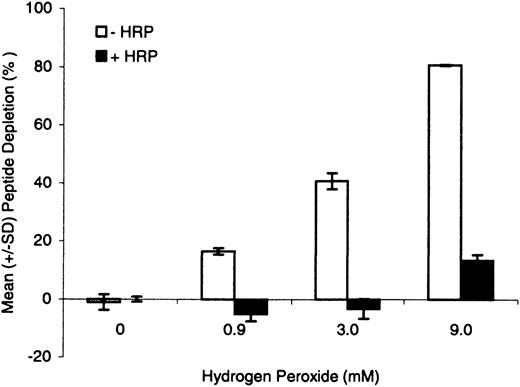
To evaluate further the apparent HRP/P-induced peptide dimerization and effectiveness of postincubation DTT treatment, loss of cysteine peptide was determined following 24 h incubation with hydrogen peroxide at 0.9, 3.0, and 9.0mM, with and without HRP. All samples were treated with DTT following the 24-h incubation period. In the absence of HRP, irreversible loss of peptide monomer was peroxide concentration-dependent with post-DTT treatment (Fig. 2). At peroxide concentrations ranging from 0.9 to 9.0mM, without HRP, irreversible depletion was 20–80%, respectively. In the presence of HRP, however, the irreversible loss was significantly attenuated, with essentially complete recovery (i.e., no measurable depletion) of peptide monomer at peroxide concentrations of 0.9 and 3.0mM. At 9.0mM peroxide, however, approximately 10% of the peptide monomer was depleted.
Cysteine peptide depletion following coincubation with increasing concentration of hydrogen peroxide for 24 h, with and without HRP (6.7 U/ml). All reactions were performed in the absence of test chemical and were treated with DTT following the 24-h incubation period. Depletion of peptide monomer is expressed in terms of mean (± SD) of triplicate incubations.
Optimization of Peroxide Concentration for Test Chemical Reactivity with Cysteine Peptide
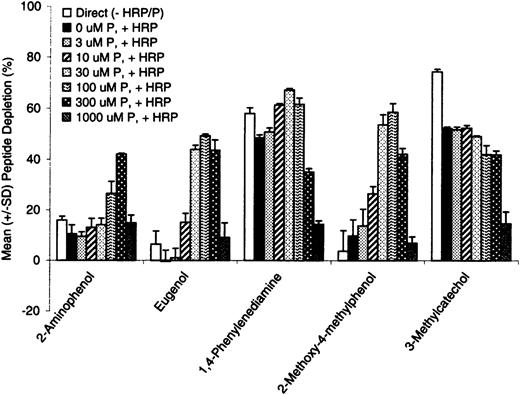
To optimize the concentration of peroxide as a cosubstrate in the assay, a dose-response study at peroxide concentrations ranging from 3 to 1000μM was examined in reactions containing 2-aminophenol, eugenol, 1,4-phenylenediamine, 2-methoxy-4-methylphenol, or 3-methylcatechol. Samples were incubated for 24 h and postincubation DTT treatment was performed to reduce any peptide dimer back to the monomeric form. In this way, lack of monomer recovery (relative to controls) could be attributed to covalent interaction with test chemical or oxidation product(s) thereof. In reactions containing HRP (6.7 U/ml), peptide depletion increased with increasing concentrations of peroxide from 3 to 100μM (for eugenol, 1,4-phenylenediamine, and 2-methoxy-4-methylphenol) or peroxide concentrations ranging from 3 to 300μM (for 2-aminophenol) (Fig. 3). As peroxide levels increased to 1000μM, peptide depletion decreased from maximum values of 40–60% to less than 15%, regardless of the test chemical examined. These findings corroborate results from preliminary studies without test chemical (described above) and suggest that the amount of available thiol for chemical reactivity is significantly reduced at peroxide concentrations > 100μM.
Test chemical reactivity to cysteine peptide and increasing concentrations of hydrogen peroxide in the presence and absence of HRP (6.7 U/ml). The figure shows depletion of peptide monomer, expressed in terms of mean (± SD) of triplicate incubations.
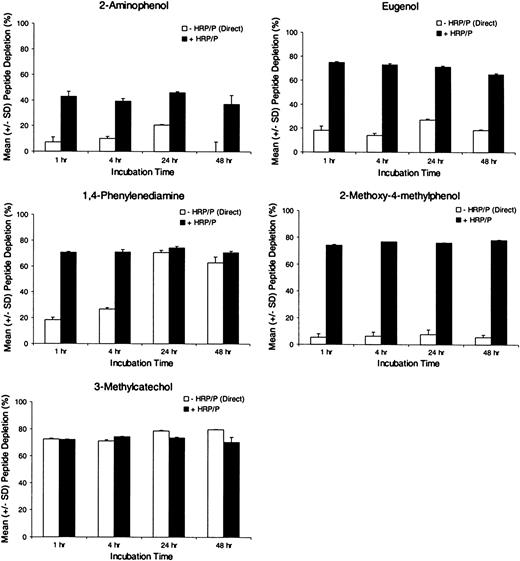
In contrast to the low nonenzymatic (direct) reactivity observed with 2-aminophenol, eugenol, and 2-methoxy-4-methylphenol, relatively high peptide depletion was observed for 1,4-phenylenediamine and 3-methylcatechol (∼60-70% depletion), suggesting a pre-hapten auto-oxidation (in air) activation mechanism. Peptide depletion profiles for 3-methylcatechol suggest no further activation in the presence of HRP/P. Because peptide depletion for pro-hapten and pre-hapten sensitizers was at or near maximum at the 100μM peroxide level, this concentration was selected for further assay development as described below.
Effect of Enzyme Concentration on Test Chemical Reactivity to Cysteine Peptide
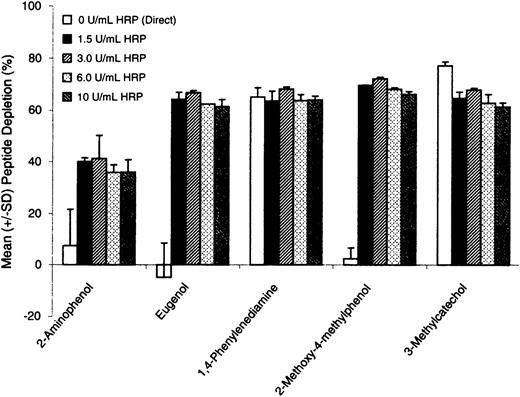
Peptide depletion in sample reactions with increasing concentrations of HRP appeared to be independent of enzyme concentration from 1.5 to 10 U/ml (Fig. 4). Consistent with previous results, nonenzymatic peptide depletion for 2-aminophenol, eugenol, and 2-methoxy-4-methylphenol was low (< 10%), whereas 1,4-phenylenediamine and 3-methylcatechol showed 65 and 77% depletion, respectively. The concentration of HRP chosen for subsequent assay development was 3.0 U/ml.
Test chemical reactivity to cysteine peptide in the absence of HRP/P (direct) and in the presence of hydrogen peroxide (100μM) and increasing concentrations of HRP. The figure shows depletion of peptide monomer, expressed in terms of mean (± SD) of triplicate incubations.
Effect of Incubation Time on Test Chemical Reactivity to Cysteine Peptide
As shown in Figure 5, peptide depletion with and without HRP/P was independent of incubation time for all chemicals examined between 1 and 24 h. An exception was the nonenzymatic reactivity for 1,4-phenylenediamine and perhaps 2-aminophenol. In addition, using relatively long incubations times (i.e., 48 h), no further depletion was observed in comparison with the 24-h time point, and for 2-aminophenol, no depletion was observed following 48 h incubation, despite approximately 20% depletion that occurred following 24 h incubation without HRP/P. The 24-h incubation time was chosen for convenience of method application and to better distinguish weak or slow reacting sensitizers from nonsensitizing chemicals that might not be discernible at shorter incubation periods (i.e., < 24 h).
Test chemical reactivity to cysteine peptide with and without HRP (3.0 U/ml) and hydrogen peroxide (100μM) following 1, 4, 24, and 48 h incubation. The figures show depletion of peptide monomer, expressed in terms of mean (± SD) of triplicate incubations.
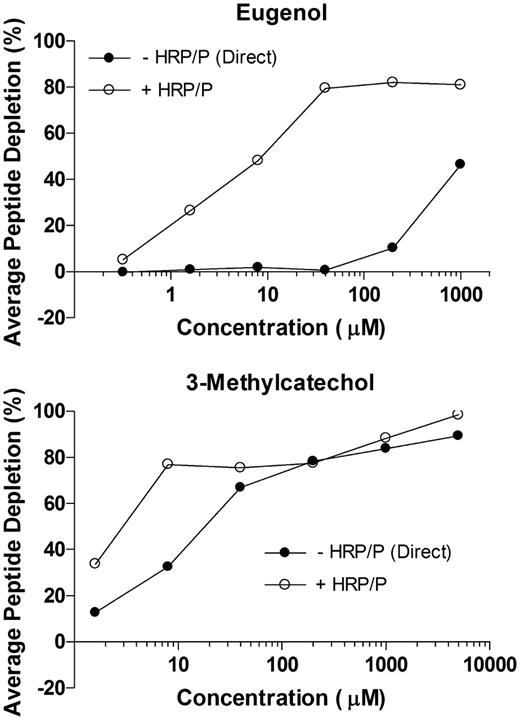
Dose-Response Studies with Eugenol and 3-Methylcatechol
Preliminary dose-response studies were conducted to characterize reactivity profiles for eugenol and 3-methylcatechol at final concentrations of 0.32, 1.6, 8.0, 40, 200, and 1000μM and 1.6, 8.0, 40, 200, 1000, and 5000μM, respectively. Concentration ranges were selected with consideration to aqueous solubility and percent peptide depletion that was previously observed at peptide and chemical concentrations of 20 and 200μM, respectively. As shown in Figure 6, peptide depletion values for eugenol increased linearly from 0.32 until reaching a plateau at 40μM in reactions with HRP/P. At eugenol concentrations of 40, 200, and 1000μM, peptide depletion values with HRP/P did not exceed approximately 80%. In contrast to incubations with enzyme, direct reactivity with eugenol was negligible (< 5%) at concentrations ≤ 40μM. As concentrations increased to 200 and 1000μM, however, depletion values increased to 10 and 46%, respectively.
Dose-response plots of eugenol and 3-methylcatechol following 24 h incubation with cysteine peptide (20μM), in the presence and absence of HRP (3.0 U/ml) and hydrogen peroxide (100μM). Sample reactions were performed at final concentrations of 0.32, 1.6, 8.0, 40, 200, and 1000μM (eugenol) or 1.6, 8.0, 40, 200, 1000, and 5000μM (3-methylcatechol). The figures show depletion of peptide monomer versus test chemical concentration, expressed in terms of the average of duplicate incubations.
In reactions with 3-methylcatechol, direct reactivity increased from 13% depletion at 1.6μM to nearly 80% depletion at 200μM. At 1000 and 5000μM, direct reactivity increased slightly to 84 and 89% depletion, respectively. Near maximal peptide depletion (∼80%) was observed at 8.0μM in the presence of HRP/P, with further increases to 88 and 98% depletion at concentrations of 1000 and 5000μM, respectively. These findings indicate that the rate of enzyme-mediated reactivity for 3-methylcatechol exceeds the formation rate of a reactive intermediate(s) in the absence of enzyme at concentrations below 200μM.
Peptide Reactivity Determinations Using Established Incubation Parameters
Peptide reactivity for test chemicals in Table 1 was determined in three independent assays using the experimental conditions that were “optimized” for peroxide (100μM), HRP (3.0 U/ml), test material concentration (200μM), incubation time (24 h), and postincubation treatment with DTT. Peptide depletion values for the nonsensitizing chemicals were generally less than 10% in reactions with or without HRP/P (Table 2). These findings corroborate results that were obtained in a single assay using the nonsensitizing chemicals, glycerol, n-hexane, and methylsalicylate (data unpublished). Enzyme-mediated activation of pro-haptens including geraniol, cinnamic alcohol, aniline, 2-aminophenol, 1-naphthol, eugenol, and 2-methoxy-4-methylphenol resulted in a significant increase in peptide depletion values with HRP/P, which ranged from approximately 10% to nearly 80%. In the absence of HRP/P, relatively little to no peptide depletion was observed. Nonenzymatic (direct) peptide depletion for pre-hapten chemical sensitizers known to auto-oxidize (hydroquinone, 4-amino-m-cresol, 3-methylcatechol, isoeugenol, and 1,4-phenylenediamine) ranged from approximately 40 to 80%. There were no significant differences observed for any of the pre-haptens with or without HRP/P. Interestingly, the nonsensitizers isopropanol and lactic acid, but not 1-butanol, yielded a significant decrease in peptide depletion in the presence of HRP/P. Finally, the results from the three independent assays for each test material indicate low interassay variability for the cysteine peptide nucleophile with and without HRP/P.
Interassay Reproducibility of the Percent Cysteine Peptide Depletion with and without HRP/Pa
| Direct (without HRP/P) | Enzyme-mediated (with HRP/P) | ||||
| Test chemical | Mean | SD | Mean | SD | |
| Nonsensitizers | Isopropanol | 9.0 | 0.1 | −0.3 | 0.9 |
| Isopropanol | 9.0 | 3.1 | 5.7 | 3.1 | |
| Isopropanol | 11.4 | 5.5 | 2.5 | 2.4 | |
| Overallb | 9.8 | 3.4 | 2.6 | 3.3 | |
| (+/−) Lactic acid | 3.3 | 3.3 | 3.9 | 0.7 | |
| (+/−) Lactic acid | 4.6 | 6.0 | 7.7 | 3.7 | |
| (+/−) Lactic acid | 19.3 | 4.5 | 1.8 | 2.6 | |
| Overallb | 9.1 | 8.7 | 4.5 | 3.4 | |
| 1-Butanol | -7.5 | 2.9 | 2.4 | 5.8 | |
| 1-Butanol | 4.2 | 0.5 | 5.6 | 2.4 | |
| 1-Butanol | 9.8 | 1.9 | 6.4 | 2.5 | |
| Overall | 2.2 | 7.8 | 4.8 | 3.8 | |
| Pro-haptens | Geraniol | -3.2 | 0.9 | 10.6 | 1.8 |
| Geraniol | 5.2 | 1.3 | 9.4 | 0.1 | |
| Geraniol | 5.3 | 2.0 | 8.1 | 1.9 | |
| Overallc | 2.4 | 4.4 | 9.3 | 1.7 | |
| Cinnamyl alcohol | −6.0 | 1.5 | 10.1 | 11.9 | |
| Cinnamyl alcohol | 2.7 | 2.0 | 10.7 | 2.3 | |
| Cinnamyl alcohol | 6.8 | 6.4 | 10.3 | 1.9 | |
| Overallc | 1.2 | 6.6 | 10.4 | 6.1 | |
| Aniline | 0.0 | 1.7 | 28.3 | 3.7 | |
| Aniline | 7.7 | 0.8 | 29.5 | 2.9 | |
| Aniline | 8.9 | 4.2 | 27.4 | 2.1 | |
| Overallc | 5.5 | 4.8 | 28.4 | 2.7 | |
| 2-Aminophenol | 15.4 | 5.0 | 42.1 | 2.1 | |
| 2-Aminophenol | 10.5 | 3.0 | 45.4 | 0.9 | |
| 2-Aminophenol | 13.4 | 0.7 | 36.3 | 3.0 | |
| Overallc | 13.1 | 3.6 | 41.3 | 4.4 | |
| 1-Naphthol | 4.8 | 2.0 | 57.0 | 6.1 | |
| 1-Naphthol | 18.5 | 1.6 | 79.9 | 3.8 | |
| 1-Naphthol | 21.9 | 0.7 | 30.3 | 1.0 | |
| Overallc | 15.0 | 8.0 | 55.8 | 21.8 | |
| Eugenol | 12.6 | 6.8 | 74.2 | 0.0 | |
| Eugenol | 12.1 | 3.8 | 65.4 | 3.1 | |
| Eugenol | 14.3 | 5.2 | 66.3 | 3.0 | |
| Overallc | 13.0 | 4.8 | 67.9 | 5.1 | |
| 2-Methoxy-4-methylphenol | 4.7 | 5.1 | 81.6 | 0.9 | |
| 2-Methoxy-4-methylphenol | 5.0 | 2.5 | 80.0 | 0.2 | |
| 2-Methoxy-4-methylphenol | 10.7 | 3.8 | 84.6 | 1.7 | |
| Overallc | 6.8 | 4.5 | 82.1 | 2.2 | |
| Pre-haptens | Hydroquinone | 46.5 | 0.8 | 42.7 | 0.5 |
| Hydroquinone | 41.9 | 3.1 | 37.3 | 1.3 | |
| Hydroquinone | 47.7 | 0.9 | 41.1 | 0.3 | |
| Overall | 45.4 | 3.1 | 40.4 | 2.5 | |
| 4-Amino-m-cresol | 54.0 | 1.8 | 39.4 | 3.8 | |
| 4-Amino-m-cresol | 55.1 | 2.2 | 46.3 | 1.6 | |
| 4-Amino-m-cresol | 56.4 | 1.8 | 44.6 | 0.6 | |
| Overall | 55.1 | 2.0 | 43.4 | 3.8 | |
| 3-Methylcatechol | 78.8 | 1.3 | 78.0 | 0.9 | |
| 3-Methylcatechol | 79.4 | 3.0 | 70.6 | 1.9 | |
| 3-Methylcatechol | 82.7 | 0.5 | 72.1 | 1.1 | |
| Overall | 80.3 | 2.5 | 73.6 | 3.6 | |
| Isoeugenol | 79.5 | 2.6 | 79.2 | 2.0 | |
| Isoeugenol | 73.6 | 1.8 | 74.4 | 1.8 | |
| Isoeugenol | 82.3 | 1.5 | 73.4 | 4.9 | |
| Overall | 78.5 | 4.2 | 75.7 | 3.9 | |
| 1,4-Phenylenediamine | 82.4 | 0.5 | 76.3 | 1.4 | |
| 1,4-Phenylenediamine | 63.8 | 0.3 | 74.9 | 0.6 | |
| 1,4-Phenylenediamine | 70.4 | 1.0 | 76.8 | 0.4 | |
| Overall | 72.2 | 8.2 | 76.0 | 1.2 | |
| Direct (without HRP/P) | Enzyme-mediated (with HRP/P) | ||||
| Test chemical | Mean | SD | Mean | SD | |
| Nonsensitizers | Isopropanol | 9.0 | 0.1 | −0.3 | 0.9 |
| Isopropanol | 9.0 | 3.1 | 5.7 | 3.1 | |
| Isopropanol | 11.4 | 5.5 | 2.5 | 2.4 | |
| Overallb | 9.8 | 3.4 | 2.6 | 3.3 | |
| (+/−) Lactic acid | 3.3 | 3.3 | 3.9 | 0.7 | |
| (+/−) Lactic acid | 4.6 | 6.0 | 7.7 | 3.7 | |
| (+/−) Lactic acid | 19.3 | 4.5 | 1.8 | 2.6 | |
| Overallb | 9.1 | 8.7 | 4.5 | 3.4 | |
| 1-Butanol | -7.5 | 2.9 | 2.4 | 5.8 | |
| 1-Butanol | 4.2 | 0.5 | 5.6 | 2.4 | |
| 1-Butanol | 9.8 | 1.9 | 6.4 | 2.5 | |
| Overall | 2.2 | 7.8 | 4.8 | 3.8 | |
| Pro-haptens | Geraniol | -3.2 | 0.9 | 10.6 | 1.8 |
| Geraniol | 5.2 | 1.3 | 9.4 | 0.1 | |
| Geraniol | 5.3 | 2.0 | 8.1 | 1.9 | |
| Overallc | 2.4 | 4.4 | 9.3 | 1.7 | |
| Cinnamyl alcohol | −6.0 | 1.5 | 10.1 | 11.9 | |
| Cinnamyl alcohol | 2.7 | 2.0 | 10.7 | 2.3 | |
| Cinnamyl alcohol | 6.8 | 6.4 | 10.3 | 1.9 | |
| Overallc | 1.2 | 6.6 | 10.4 | 6.1 | |
| Aniline | 0.0 | 1.7 | 28.3 | 3.7 | |
| Aniline | 7.7 | 0.8 | 29.5 | 2.9 | |
| Aniline | 8.9 | 4.2 | 27.4 | 2.1 | |
| Overallc | 5.5 | 4.8 | 28.4 | 2.7 | |
| 2-Aminophenol | 15.4 | 5.0 | 42.1 | 2.1 | |
| 2-Aminophenol | 10.5 | 3.0 | 45.4 | 0.9 | |
| 2-Aminophenol | 13.4 | 0.7 | 36.3 | 3.0 | |
| Overallc | 13.1 | 3.6 | 41.3 | 4.4 | |
| 1-Naphthol | 4.8 | 2.0 | 57.0 | 6.1 | |
| 1-Naphthol | 18.5 | 1.6 | 79.9 | 3.8 | |
| 1-Naphthol | 21.9 | 0.7 | 30.3 | 1.0 | |
| Overallc | 15.0 | 8.0 | 55.8 | 21.8 | |
| Eugenol | 12.6 | 6.8 | 74.2 | 0.0 | |
| Eugenol | 12.1 | 3.8 | 65.4 | 3.1 | |
| Eugenol | 14.3 | 5.2 | 66.3 | 3.0 | |
| Overallc | 13.0 | 4.8 | 67.9 | 5.1 | |
| 2-Methoxy-4-methylphenol | 4.7 | 5.1 | 81.6 | 0.9 | |
| 2-Methoxy-4-methylphenol | 5.0 | 2.5 | 80.0 | 0.2 | |
| 2-Methoxy-4-methylphenol | 10.7 | 3.8 | 84.6 | 1.7 | |
| Overallc | 6.8 | 4.5 | 82.1 | 2.2 | |
| Pre-haptens | Hydroquinone | 46.5 | 0.8 | 42.7 | 0.5 |
| Hydroquinone | 41.9 | 3.1 | 37.3 | 1.3 | |
| Hydroquinone | 47.7 | 0.9 | 41.1 | 0.3 | |
| Overall | 45.4 | 3.1 | 40.4 | 2.5 | |
| 4-Amino-m-cresol | 54.0 | 1.8 | 39.4 | 3.8 | |
| 4-Amino-m-cresol | 55.1 | 2.2 | 46.3 | 1.6 | |
| 4-Amino-m-cresol | 56.4 | 1.8 | 44.6 | 0.6 | |
| Overall | 55.1 | 2.0 | 43.4 | 3.8 | |
| 3-Methylcatechol | 78.8 | 1.3 | 78.0 | 0.9 | |
| 3-Methylcatechol | 79.4 | 3.0 | 70.6 | 1.9 | |
| 3-Methylcatechol | 82.7 | 0.5 | 72.1 | 1.1 | |
| Overall | 80.3 | 2.5 | 73.6 | 3.6 | |
| Isoeugenol | 79.5 | 2.6 | 79.2 | 2.0 | |
| Isoeugenol | 73.6 | 1.8 | 74.4 | 1.8 | |
| Isoeugenol | 82.3 | 1.5 | 73.4 | 4.9 | |
| Overall | 78.5 | 4.2 | 75.7 | 3.9 | |
| 1,4-Phenylenediamine | 82.4 | 0.5 | 76.3 | 1.4 | |
| 1,4-Phenylenediamine | 63.8 | 0.3 | 74.9 | 0.6 | |
| 1,4-Phenylenediamine | 70.4 | 1.0 | 76.8 | 0.4 | |
| Overall | 72.2 | 8.2 | 76.0 | 1.2 | |
Reactivity of test chemicals to cysteine peptide at a peptide:chemical ratio of 1:10 with and without HRP (3.0 U/ml) and hydrogen peroxide (100μM) following 24 h incubation. Results are expressed as the mean ± SD of percent depletion of nonreacted peptide from three discrete experiments. The overall mean ± SD of percent depletion from all experiments is also presented.
A significant decrease in peptide depletion with HRP/P was observed in comparison with peptide depletion without HRP/P (p < 0.05).
A significant increase in peptide depletion with HRP/P was observed in comparison with peptide depletion without HRP/P (p < 0.05).
Interassay Reproducibility of the Percent Cysteine Peptide Depletion with and without HRP/Pa
| Direct (without HRP/P) | Enzyme-mediated (with HRP/P) | ||||
| Test chemical | Mean | SD | Mean | SD | |
| Nonsensitizers | Isopropanol | 9.0 | 0.1 | −0.3 | 0.9 |
| Isopropanol | 9.0 | 3.1 | 5.7 | 3.1 | |
| Isopropanol | 11.4 | 5.5 | 2.5 | 2.4 | |
| Overallb | 9.8 | 3.4 | 2.6 | 3.3 | |
| (+/−) Lactic acid | 3.3 | 3.3 | 3.9 | 0.7 | |
| (+/−) Lactic acid | 4.6 | 6.0 | 7.7 | 3.7 | |
| (+/−) Lactic acid | 19.3 | 4.5 | 1.8 | 2.6 | |
| Overallb | 9.1 | 8.7 | 4.5 | 3.4 | |
| 1-Butanol | -7.5 | 2.9 | 2.4 | 5.8 | |
| 1-Butanol | 4.2 | 0.5 | 5.6 | 2.4 | |
| 1-Butanol | 9.8 | 1.9 | 6.4 | 2.5 | |
| Overall | 2.2 | 7.8 | 4.8 | 3.8 | |
| Pro-haptens | Geraniol | -3.2 | 0.9 | 10.6 | 1.8 |
| Geraniol | 5.2 | 1.3 | 9.4 | 0.1 | |
| Geraniol | 5.3 | 2.0 | 8.1 | 1.9 | |
| Overallc | 2.4 | 4.4 | 9.3 | 1.7 | |
| Cinnamyl alcohol | −6.0 | 1.5 | 10.1 | 11.9 | |
| Cinnamyl alcohol | 2.7 | 2.0 | 10.7 | 2.3 | |
| Cinnamyl alcohol | 6.8 | 6.4 | 10.3 | 1.9 | |
| Overallc | 1.2 | 6.6 | 10.4 | 6.1 | |
| Aniline | 0.0 | 1.7 | 28.3 | 3.7 | |
| Aniline | 7.7 | 0.8 | 29.5 | 2.9 | |
| Aniline | 8.9 | 4.2 | 27.4 | 2.1 | |
| Overallc | 5.5 | 4.8 | 28.4 | 2.7 | |
| 2-Aminophenol | 15.4 | 5.0 | 42.1 | 2.1 | |
| 2-Aminophenol | 10.5 | 3.0 | 45.4 | 0.9 | |
| 2-Aminophenol | 13.4 | 0.7 | 36.3 | 3.0 | |
| Overallc | 13.1 | 3.6 | 41.3 | 4.4 | |
| 1-Naphthol | 4.8 | 2.0 | 57.0 | 6.1 | |
| 1-Naphthol | 18.5 | 1.6 | 79.9 | 3.8 | |
| 1-Naphthol | 21.9 | 0.7 | 30.3 | 1.0 | |
| Overallc | 15.0 | 8.0 | 55.8 | 21.8 | |
| Eugenol | 12.6 | 6.8 | 74.2 | 0.0 | |
| Eugenol | 12.1 | 3.8 | 65.4 | 3.1 | |
| Eugenol | 14.3 | 5.2 | 66.3 | 3.0 | |
| Overallc | 13.0 | 4.8 | 67.9 | 5.1 | |
| 2-Methoxy-4-methylphenol | 4.7 | 5.1 | 81.6 | 0.9 | |
| 2-Methoxy-4-methylphenol | 5.0 | 2.5 | 80.0 | 0.2 | |
| 2-Methoxy-4-methylphenol | 10.7 | 3.8 | 84.6 | 1.7 | |
| Overallc | 6.8 | 4.5 | 82.1 | 2.2 | |
| Pre-haptens | Hydroquinone | 46.5 | 0.8 | 42.7 | 0.5 |
| Hydroquinone | 41.9 | 3.1 | 37.3 | 1.3 | |
| Hydroquinone | 47.7 | 0.9 | 41.1 | 0.3 | |
| Overall | 45.4 | 3.1 | 40.4 | 2.5 | |
| 4-Amino-m-cresol | 54.0 | 1.8 | 39.4 | 3.8 | |
| 4-Amino-m-cresol | 55.1 | 2.2 | 46.3 | 1.6 | |
| 4-Amino-m-cresol | 56.4 | 1.8 | 44.6 | 0.6 | |
| Overall | 55.1 | 2.0 | 43.4 | 3.8 | |
| 3-Methylcatechol | 78.8 | 1.3 | 78.0 | 0.9 | |
| 3-Methylcatechol | 79.4 | 3.0 | 70.6 | 1.9 | |
| 3-Methylcatechol | 82.7 | 0.5 | 72.1 | 1.1 | |
| Overall | 80.3 | 2.5 | 73.6 | 3.6 | |
| Isoeugenol | 79.5 | 2.6 | 79.2 | 2.0 | |
| Isoeugenol | 73.6 | 1.8 | 74.4 | 1.8 | |
| Isoeugenol | 82.3 | 1.5 | 73.4 | 4.9 | |
| Overall | 78.5 | 4.2 | 75.7 | 3.9 | |
| 1,4-Phenylenediamine | 82.4 | 0.5 | 76.3 | 1.4 | |
| 1,4-Phenylenediamine | 63.8 | 0.3 | 74.9 | 0.6 | |
| 1,4-Phenylenediamine | 70.4 | 1.0 | 76.8 | 0.4 | |
| Overall | 72.2 | 8.2 | 76.0 | 1.2 | |
| Direct (without HRP/P) | Enzyme-mediated (with HRP/P) | ||||
| Test chemical | Mean | SD | Mean | SD | |
| Nonsensitizers | Isopropanol | 9.0 | 0.1 | −0.3 | 0.9 |
| Isopropanol | 9.0 | 3.1 | 5.7 | 3.1 | |
| Isopropanol | 11.4 | 5.5 | 2.5 | 2.4 | |
| Overallb | 9.8 | 3.4 | 2.6 | 3.3 | |
| (+/−) Lactic acid | 3.3 | 3.3 | 3.9 | 0.7 | |
| (+/−) Lactic acid | 4.6 | 6.0 | 7.7 | 3.7 | |
| (+/−) Lactic acid | 19.3 | 4.5 | 1.8 | 2.6 | |
| Overallb | 9.1 | 8.7 | 4.5 | 3.4 | |
| 1-Butanol | -7.5 | 2.9 | 2.4 | 5.8 | |
| 1-Butanol | 4.2 | 0.5 | 5.6 | 2.4 | |
| 1-Butanol | 9.8 | 1.9 | 6.4 | 2.5 | |
| Overall | 2.2 | 7.8 | 4.8 | 3.8 | |
| Pro-haptens | Geraniol | -3.2 | 0.9 | 10.6 | 1.8 |
| Geraniol | 5.2 | 1.3 | 9.4 | 0.1 | |
| Geraniol | 5.3 | 2.0 | 8.1 | 1.9 | |
| Overallc | 2.4 | 4.4 | 9.3 | 1.7 | |
| Cinnamyl alcohol | −6.0 | 1.5 | 10.1 | 11.9 | |
| Cinnamyl alcohol | 2.7 | 2.0 | 10.7 | 2.3 | |
| Cinnamyl alcohol | 6.8 | 6.4 | 10.3 | 1.9 | |
| Overallc | 1.2 | 6.6 | 10.4 | 6.1 | |
| Aniline | 0.0 | 1.7 | 28.3 | 3.7 | |
| Aniline | 7.7 | 0.8 | 29.5 | 2.9 | |
| Aniline | 8.9 | 4.2 | 27.4 | 2.1 | |
| Overallc | 5.5 | 4.8 | 28.4 | 2.7 | |
| 2-Aminophenol | 15.4 | 5.0 | 42.1 | 2.1 | |
| 2-Aminophenol | 10.5 | 3.0 | 45.4 | 0.9 | |
| 2-Aminophenol | 13.4 | 0.7 | 36.3 | 3.0 | |
| Overallc | 13.1 | 3.6 | 41.3 | 4.4 | |
| 1-Naphthol | 4.8 | 2.0 | 57.0 | 6.1 | |
| 1-Naphthol | 18.5 | 1.6 | 79.9 | 3.8 | |
| 1-Naphthol | 21.9 | 0.7 | 30.3 | 1.0 | |
| Overallc | 15.0 | 8.0 | 55.8 | 21.8 | |
| Eugenol | 12.6 | 6.8 | 74.2 | 0.0 | |
| Eugenol | 12.1 | 3.8 | 65.4 | 3.1 | |
| Eugenol | 14.3 | 5.2 | 66.3 | 3.0 | |
| Overallc | 13.0 | 4.8 | 67.9 | 5.1 | |
| 2-Methoxy-4-methylphenol | 4.7 | 5.1 | 81.6 | 0.9 | |
| 2-Methoxy-4-methylphenol | 5.0 | 2.5 | 80.0 | 0.2 | |
| 2-Methoxy-4-methylphenol | 10.7 | 3.8 | 84.6 | 1.7 | |
| Overallc | 6.8 | 4.5 | 82.1 | 2.2 | |
| Pre-haptens | Hydroquinone | 46.5 | 0.8 | 42.7 | 0.5 |
| Hydroquinone | 41.9 | 3.1 | 37.3 | 1.3 | |
| Hydroquinone | 47.7 | 0.9 | 41.1 | 0.3 | |
| Overall | 45.4 | 3.1 | 40.4 | 2.5 | |
| 4-Amino-m-cresol | 54.0 | 1.8 | 39.4 | 3.8 | |
| 4-Amino-m-cresol | 55.1 | 2.2 | 46.3 | 1.6 | |
| 4-Amino-m-cresol | 56.4 | 1.8 | 44.6 | 0.6 | |
| Overall | 55.1 | 2.0 | 43.4 | 3.8 | |
| 3-Methylcatechol | 78.8 | 1.3 | 78.0 | 0.9 | |
| 3-Methylcatechol | 79.4 | 3.0 | 70.6 | 1.9 | |
| 3-Methylcatechol | 82.7 | 0.5 | 72.1 | 1.1 | |
| Overall | 80.3 | 2.5 | 73.6 | 3.6 | |
| Isoeugenol | 79.5 | 2.6 | 79.2 | 2.0 | |
| Isoeugenol | 73.6 | 1.8 | 74.4 | 1.8 | |
| Isoeugenol | 82.3 | 1.5 | 73.4 | 4.9 | |
| Overall | 78.5 | 4.2 | 75.7 | 3.9 | |
| 1,4-Phenylenediamine | 82.4 | 0.5 | 76.3 | 1.4 | |
| 1,4-Phenylenediamine | 63.8 | 0.3 | 74.9 | 0.6 | |
| 1,4-Phenylenediamine | 70.4 | 1.0 | 76.8 | 0.4 | |
| Overall | 72.2 | 8.2 | 76.0 | 1.2 | |
Reactivity of test chemicals to cysteine peptide at a peptide:chemical ratio of 1:10 with and without HRP (3.0 U/ml) and hydrogen peroxide (100μM) following 24 h incubation. Results are expressed as the mean ± SD of percent depletion of nonreacted peptide from three discrete experiments. The overall mean ± SD of percent depletion from all experiments is also presented.
A significant decrease in peptide depletion with HRP/P was observed in comparison with peptide depletion without HRP/P (p < 0.05).
A significant increase in peptide depletion with HRP/P was observed in comparison with peptide depletion without HRP/P (p < 0.05).
DISCUSSION
In an effort to address the forthcoming European Union ban on in vivo testing of cosmetics and toiletry ingredients, investigators have been developing alternative methodologies for skin sensitization using in vitro, in silico, and in chemico approaches. Specifically, investigators have been promoting the development of quantitative peptide-based reactivity assays for screening chemical allergens (Aleksic et al., 2009; Aptula et al., 2006; Gerberick et al., 2004, 2007; Kato et al., 2003; Natsch and Gfeller, 2008; Natsch et al., 2007; Roberts and Natsch, 2009; Schultz et al., 2005). One limitation of the approaches evaluated to date is that pro-haptens and some pre-haptens are not detected as reactive compounds because the methods do not allow for either spontaneous oxidation or metabolic activation. In this paper we have evaluated the utility of using HRP/P for the specific activation of pro-haptens.
It has been estimated that up to one third of known sensitizers are not directly reactive and thus require some form of activation prior to reacting with proteins containing nucleophilic amino acids (Smith and Hotchkiss, 2001). The skin sensitizers selected for this investigation were chosen because they are nonelectrophilic and require activation to form protein reactive intermediates (Table 1). Aniline, geraniol, cinnamyl alcohol, eugenol, 2-methoxy-4-methylphenol, 1-napthol, and 2-aminophenol are considered pro-haptens, whereas 4-amino-m-cresol, isoeugenol, 1,4-phenylenediamine, hydroquinone, and 3-methylcatechol could be classified as pre-haptens and are known to oxidize easily following exposure to atmospheric oxygen (Gerberick et al., 2008; Smith and Hotchkiss, 2001; Smith-Pease et al., 2003). Additionally, these compounds encompass a broad range of sensitization potencies including four extreme/strong, four moderate, and four weak as well as three nonsensitizers. Their chemical structures include aliphatic alcohols and various hydroxylated or aminated aromatic derivatives which are likely representative of different chemical reaction mechanisms evidenced for modifying proteins (e.g., Michael acceptor, SN2 electrophiles) (Roberts et al., 2007).
To detect the reactivity of pre- and pro-haptens, we investigated the utility of using HRP/P as a pragmatic enzymatic system. HRP catalyzes the conversion (via oxidation) of the nonreactive chemical sensitizer to a reactive (electrophilic) intermediate which can then form adducts with a nucleophile-containing peptide. HRP is a classic peroxidase that has been used for characterizing and comparing the peroxidatic activity of other xenobiotic-metabolizing enzymes (e.g., cytochrome P450 [P450], myeloperoxidase, catalase, cyclooxygenase) (Marnett et al., 1986; Testa, 1995). Unlike some peroxidases that are limited in regard to the chemicals that function as electron donors or acceptors, HRP has an accessible active site that enables activation of structurally diverse pro-hapten substrates.
Due to the oxidative environment imparted by HRP/P, and propensity of the thiol-containing cysteine peptide to readily dimerize, studies were conducted to evaluate recovery of the peptide following incubation with and without HRP/P, in the absence of any test chemical. Coincubation of cysteine peptide with HRP and hydrogen peroxide for 2 or 24 h resulted in virtually the complete loss of the peptide monomer and extensive dimer formation (Fig. 1). In incubations without HRP/P, depletion of peptide monomer and formation of dimer occurred to a much less extent than samples containing HRP/P. Subsequent treatment of the 24-h samples with DTT (post-DTT) resulted in the reduction of the disulfide bond in thiol-dimers with conversion of the peptide back to the monomeric form. Thus, DTT is an effective agent for reversing the apparent dimerization of the thiol groups in the cysteine peptide nucleophile.
Coincubation of test chemicals with HRP at higher peroxide concentrations (> 100μM) showed an apparent increase in the rate of peptide dimerization and/or oxidation that exceeds the rate of peptide-chemical reactivity during the incubation period (Figs. 2 and 3). Even though the postincubation DTT treatment converts the peptide dimer back to its monomeric form, the net measurement for peptide depletion may be negligible relative to control due to rapid dimerization at these higher peroxide levels. In the absence of HRP, irreversible loss of peptide monomer was peroxide concentration-dependent with post-DTT treatment. At peroxide concentrations ranging from 0.9 to 9.0mM, without HRP, irreversible loss of monomer was observed with depletion values ranging from 20 to 80%, respectively. In the presence of HRP, however, the irreversible loss was attenuated, with essentially complete recovery of peptide monomer at peroxide concentrations of 0.9 and 3.0mM. At 9.0mM peroxide, approximately 10% of the peptide monomer was depleted. Thus, the concentration of hydrogen peroxide required for enzyme-mediated reactivity determinations must be kept below 9.0mM to minimize the oxidative effects of the hydrogen peroxide. Based on peptide reactivity determinations that were conducted with prototypic pre- and pro-hapten sensitizers, 100μM was selected as the optimal concentration of hydrogen peroxide.
To optimize further the conditions for using HRP/P, the optimal incubation kinetics and HRP concentration was evaluated. The effect of HRP enzyme concentration on test chemical reactivity to cysteine peptide was similar at concentrations ranging from 1.5 to 10 U/ml (Fig. 4). Peptide depletion for all chemicals tested with HRP yielded similar peptide depletion results between 1- and 24-h incubations (Fig. 5). Interestingly, for 2-aminophenol and 1,4-phenylenediamine longer incubation times (i.e., 24 h) allowed for more peptide depletion for nonenzymatic reactions relative to those at 1 and 4 h. From these studies, 3.0 U/ml HRP and 24-h incubation time were chosen for subsequent experimental studies.
The HRP/P enzymatic system provides a straightforward approach for detecting the peptide reactivity of many well-known pro-haptens via an enzyme-mediated oxidative process (Table 2). HRP is relatively inexpensive, readily available, well characterized and highly stable. Moreover, its open active site is less encumbered by substrate specificity limitations as compared with CYP-isoform specific enzymes. Peroxidase-dependent activation is an important activation step for xenobiotics in the skin (Gibbs et al., 2007), and mediating enzymes such as myeloperoxidase and cyclooxygenase are expressed in dendritic cells (Chen et al., 2004; Je et al., 2008). A possible limitation of only using HRP/P is that some pro-haptens may require enzyme activation by specific enzymes (e.g., P450, alcohol dehydrogenase) or a combination thereof. Thus, it has to be explored further whether pro-haptens requiring specific enzymatic activation can be detected with HRP/P. It is important to note that the metabolic capability of the skin is complex and not well characterized (Gibbs et al., 2007), so validated systems with relevant enzymes have not been identified for use in in vitro/in chemico methods for skin sensitization testing. Experiments conducted in our laboratory with S9 demonstrated that the reactivity of some pro-haptens was not detected, possibly due to detoxification of chemicals via phase II enzymes or adduct formation to molecules that are endogenous to the in vitro system (e.g., S9 protein) (data not shown). One promising approach has been proposed by Bergström et al. (2007) who developed “skin-like” cocktails of metabolizing enzymes for use in investigating the reactivity of sensitizers using a panel of single nucleophile peptides. Additional evaluation of the HRP/P approach with numerous pro- and pre-haptens will be needed to determine the utility of HRP/P.
The overall advantage of using HRP/P oxidation system is that it is simple and allows for determination and comparison of both direct and enzyme-mediated reactivity in a single experiment. We have found that up to 32 test chemicals can be conveniently analyzed, in triplicate, within a single batch run. With the use of a 96-well layout, automated batch handling and rapid HPLC/MS/MS analysis, peptide reactivity determinations for 32 test chemicals can be achieved within a 3-day period. Due to the use of the sensitive and selective HPLC/MS/MS detection system, relatively low concentrations of the reactants are required, which ultimately reduce limitations due to insufficient solubility of test chemicals. In comparison with other detection systems (e.g., LC/UV), there is also less potential for interferences from test chemical or metabolites. The percent peptide depletion of pro-haptens aniline, 2-aminophenol, 1-napthol, eugenol, and 2-methoxy-4-methylphenol was significantly increased in the presence of HRP/P, thus demonstrating the enzymatic activation of the pro-haptens to reactive chemicals. Although significant, the two aliphatic alcohols, geraniol and cinnamic alcohol, demonstrated a much less increase in percent peptide depletion. Because aldehydes can be easily oxidized to carboxylic acids, it is possible that the low depletion is actually due to deactivation of some of the reactive aldehyde with conversion to the nonreactive acid. Possibly the reactivity of these two weak allergens would be increased if higher test concentrations or a different enzyme system were used to enhance adduct formation. The percent peptide depletion of pre-haptens hydroquinone, 4-amino-m-cresol, 3-methylcatechol, isoeugenol, and 1,4-phenylenediamine was equally high with and without the incorporation of HRP/P. Although significant differences in depletion between direct and enzyme-mediated reactions with isopropanol and (+/−) lactic acid were observed, it is important to note that peptide depletion without HRP/P was generally low (< 10%). Upon coincubation with HRP/P, depletion values actually decreased relative to those observed under direct reactivity determinations. In comparison, all pro-haptens were associated with a significant increase in depletion with HRP/P.
The goal of this work was to evaluate the use of HRP/P oxidation systems for the determination of peptide reactivity of pro-haptens. Results from this preliminary work highlight the utility of using HRP/P in chemico for screening potential pro-hapten chemical sensitizers. With further refinements, such as dose-response testing with additional nucleophiles, and/or enzyme systems, a more comprehensive approach for detecting the reactivity of all skin allergens including haptens, pro-haptens, and pre-haptens may be possible.
FUNDING
This work is funded in part by The European Cosmetics Association.
The authors wish to acknowledge George Daston, Cindy Ryan, and Petra Kern for their excellent review of the manuscript and Pam Riley for her technical contributions to this work. We also acknowledge Joel Chaney for his assistance on the statistical procedures used in this manuscript. None of the P&G authors will benefit financially from this work other than their salaries. Dr Lepoittevin works in collaboration with the P&G scientists and is not receiving any financial support from P&G for this work.




Comments