-
PDF
- Split View
-
Views
-
Cite
Cite
Jack Stack, John-Paul Hodnett, Spencer G Lucas, Lauren Sallan, Tanyrhinichthys mcallisteri, a long-rostrumed Pennsylvanian ray-finned fish (Actinopterygii) and the simultaneous appearance of novel ecomorphologies in Late Palaeozoic fishes, Zoological Journal of the Linnean Society, Volume 191, Issue 2, February 2021, Pages 347–374, https://doi.org/10.1093/zoolinnean/zlaa044
Close - Share Icon Share
Abstract
The Carboniferous radiation of fishes was marked by the convergent appearance of then-novel but now common ecomorphologies resulting from changes in the relative proportions of traits, including elongation of the front of the skull (rostrum). The earliest ray-finned fishes (Actinopterygii) with elongate rostra are poorly known, obscuring the earliest appearances of a now widespread feature in actinopterygians. We redescribe Tanyrhinichthys mcallisteri, a long-rostrumed actinopterygian from the Upper Pennsylvanian (Missourian) of the Kinney Brick Quarry, New Mexico. Tanyrhinichthys has a lengthened rostrum bearing a sensory canal, ventrally inserted paired fins, posteriorly placed median fins unequal in size and shape, and a heterocercal caudal fin. Tanyrhinichthys shares these features with sturgeons, but lacks chondrostean synapomorphies, indicating convergence on a bottom-feeding lifestyle. Elongate rostra evolved independently in two lineages of bottom-dwelling, freshwater actinopterygians in the Late Pennsylvanian of Euramerica, as well as in at least one North American chondrichthyan (Bandringa rayi). The near-simultaneous appearance of novel ecomorphologies among multiple, distantly related lineages of actinopterygians and chondrichthyans was common during the Carboniferous radiation of fishes. This may reflect global shifts in marine and freshwater ecosystems and environments during the Carboniferous favouring such ecomorphologies, or it may have been contingent on the plasticity of early actinopterygians and chondrichthyans.
INTRODUCTION
The Carboniferous is defined by large diversification events among fishes and tetrapods following the end-Devonian mass extinction (359 Mya; Sallan & Coates, 2010; Sallan & Galimberti, 2015). This led to the establishment of the first ecosystems with faunas dominated by ray-finned fishes and chondrichthyans in both marine and freshwater settings, many exhibiting ecomorphologies shared with extant fishes (Sallan & Coates, 2010, 2013; Sallan & Friedman, 2012). Characterizing the historical patterns and evolutionary processes that drove these diversification events will require a thorough understanding of the ecomorphology of Carboniferous fishes worldwide. Unfortunately, while the ecological and taxonomic composition of Carboniferous fish and tetrapod faunas from the UK, Central Europe, eastern North America and elsewhere have received renewed attention of late, the south-western US remains relatively neglected and poorly described, despite abundant Palaeozoic material (Kues & Lucas, 1992; Hodnett & Lucas, 2015).
This study is part of a larger effort to collect from and document the bountiful Late Palaeozoic faunas from the United States. These faunas include the Kinney Brick Quarry (KBQ), a source of abundant Carboniferous fossils that preserves an ancient estuary from the Late Pennsylvanian (Missourian, approximately 303.7–306 Myr old) of New Mexico (Lucas et al., 2011). KBQ contains an uncommon, mostly non-marine assemblage of diverse and well-preserved fishes from the Tinajas Member of the Atrasado Formation (Kues & Lucas, 1992; Lucas et al., 2011; Williams & Lucas, 2013). While actinopterygian fossils are common, sharks and coelacanths are rare but diverse within the KBQ fish fauna (Zidek, 1992). The excellent degree of preservation of an entire assemblage of fishes, together with the rest of the KBQ Lagerstätte, is a rare opportunity to study the morphology and palaeoecology of Late Pennsylvanian fishes in depth (Kues & Lucas, 1992; Williams & Lucas, 2013). The study of the KBQ fish fauna contributes to the body of knowledge that will be required to understand the Carboniferous diversification of fishes.
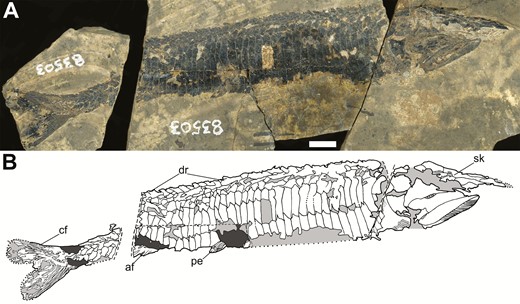
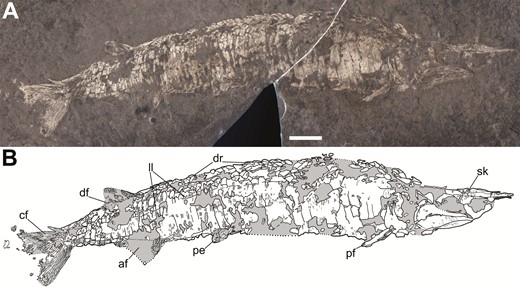
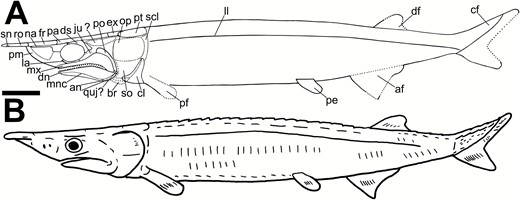
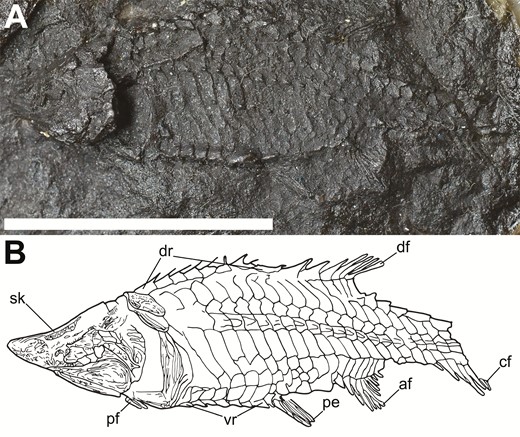
Tanyrhinichthys mcallisteri (Gottfried, 1987) is a small actinopterygian from KBQ previously known only from the holotype, KUVP 83503, collected as part of a larger group of fish fossils by a 1984 University of Kansas expedition to KBQ (Gottfried, 1987). KUVP 83503 has a badly crushed skull, an incomplete tail and nearly or completely lacks much of the median and paired fins (Gottfried, 1987; Fig. 1). Tanyrhinichthys was inferred to be morphologically convergent on the ram-feeding ambush predator morphotype of pike and gar described by Webb (1984a) (Gottfried, 1987). Since the initial description, five new specimens (NMMNH P-51192, NMMNH P-70413, NMMNH P-70411, NMMNH P-67687 and CM 30737) have been recovered, including the only complete specimen of Tanyrhinichthys (Hodnett & Lucas, 2015; CM 30737, Fig. 2). These specimens provide new information on structures that were poorly preserved in the holotype, most notably the skull (CM 30737, P-70413 and P-51192), the pectoral fins (CM 30737, P-70413 and P-51192), the overall shape of the body (CM 30737 and P-70413), the dorsal fin (CM 30737), the anal fin (CM 30737) and the caudal fin (CM 30737 and P-51192). Examination of these new specimens and re-evaluation of KUVP 83503 forms the basis for a thorough revision of Tanyrhinichthys.
The holotype of Tanyrhinichthys, KUVP 83503 (anterior is to the right). A, specimen photo. B, specimen drawing. Scale bar equals 1 cm.
The most complete specimen of Tanyrhinichthys, CM 30737 (anterior is to the right). A, specimen photo (colour inverted). B, specimen drawing. Scale bar equals 1 cm.
Tanyrhinichthys is one of several poorly known long-rostrumed Palaeozoic actinopterygians, including two other freshwater forms from a brief interval in the Late Carboniferous. The most complete of these fishes is Phanerorhynchus armatus (Gill, 1923), which is known from a single specimen (L. 8585) from the Pennsylvanian of the Middle Coal Measures at Sparth, near Rochdale, UK (Gill, 1923). Poplin (1978) also documented a skull roof (PF 2289) of an undescribed long-rostrumed actinopterygian from the Pennsylvanian of Logan Quarry, Indiana. Additionally, two other long-rostrumed actinopterygians are known from other parts of the Palaeozoic. Tegeolepis clarki (Newberry, 1888) from the Cleveland Shale Member of the Upper Devonian (Famennian) Ohio Shale (Ohio, USA) (Dunkle & Schaeffer, 1973) and Eosaurichthys chaoi (a possible junior synonym of Saurichthys; see: Tintori, 2013; Liu & Wei, 1988) from the latest Permian (Changhsingian) of Zhejiang, China, bear elongate rostra. We compare our revised description of Tanyrhinichthys with previous descriptions of Phanerorhynchus (Gill 1923; Gardiner 1967), Tegeolepis (Gardiner, 1963; Dunkle & Schaeffer, 1973), Eosaurichthys (Liu & Wei, 1988) and the unnamed taxon from Indiana (Poplin, 1978) to determine the extent of the similarity between these taxa and make inferences regarding the early evolutionary history of elongate rostra in ray-finned fishes.
We redescribe the morphology of Tanyrhinichthys and create a more complete and accurate reconstruction of this fish as a living animal. We compare our reconstruction to modern analogues to re-evaluate the hypothesized palaeoecology of Tanyrhinichthys. We also compare Tanyrhinichthys to other long-rostrumed Palaeozoic actinopterygians to examine its potential evolutionary relationships and the evolution of elongate rostra amongst Palaeozoic ray-finned fishes. Finally, we review other novel morphologies that arose in Carboniferous fishes to place Tanyrhinichthys into the broader context of ecomorphological evolution and diversification in the aftermath of the end-Devonian Hangenberg event.
MATERIAL AND METHODS
All catalogued specimens of Tanyrhinichthys from the New Mexico Museum of Natural History and Science (NMMNH), the University of Kansas Museum of Natural History (KUVP) and the Carnegie Museum of Natural History (CM) were examined, drawn and photographed. In our interpretative drawings, dotted lines indicate inferred boundaries, dashed lines show physical breaks in the rock, light grey infill marks areas within the specimen where bone is absent and dark grey infill marks areas where the bone is degraded to the point where reliable identification of individual elements is not possible. The colour of the photographs of CM-30737 was inverted in Adobe Photoshop CC to make details of the bones clearly visible. New specimens of Tanyrhinichthys were compared to KUVP 83503 and used to determine what previously undescribed features are preserved. The morphology of the new specimens of Tanyrhinichthys was then compared to modern analogues to make inferences regarding its ecology.
We compared our redescription of Tanyrhinichthys to published descriptions of other Palaeozoic taxa with lengthened or enlarged rostra (Gill, 1923; Gardiner, 1967; Dunkle & Schaeffer, 1973; Poplin, 1978; Schultze & Bardack, 1987; Liu & Wei, 1988). JS also examined and photographed silicone-rubber peels of the holotype of Phanerorhynchus (P. 34421–2 and P. 50023–4) at the Natural History Museum, London, UK (NHM) and the holotype of Illinichthys cozarti (UC 21716) at the Field Museum of Natural History, Chicago, USA (FMNH). MicroCT scans of the holotype of Phanerorhynchus (L. 8585, deposited in the Manchester Museum, UK), provided by Matt Friedman, were also used for comparisons. These scans were conducted at the CTEES facility at the University of Michigan using a Nikon XT H 225 ST scanner. The parameters of the scan were as follows: resolution (26.6 microns), voltage (210 kV), current (235 uA), filter (2 mm Cu), projections (3141, one frame per second) and exposure time (1415 ms). The specimen (PF 2289), upon which Poplin’s (1978) description of a long-rostrumed taxa from the Logan Quarry of Indiana is based, could not be located by JS at the FMNH. Figures were rendered using Adobe Photoshop CC from specimen photos scanned at 1200 dpi on an Epson Perfection V600 scanner. The photograph in Figure 7 was taken with a Leica DFC495 microscope camera mounted on a Leica DFC495 microscope and the photograph in Figure 15 was taken with a Nikon D7000 camera with a 105.0 mm f/2.8 macro lens.
Bone nomenclature follows the conventional terminology for actinopterygians (Gardiner, 1984) to facilitate comparisons to previous publications. In this terminology, the frontals and parietals of actinopterygians are homologous to the parietals and postparietals of sarcopterygians (Schultze, 2008).
Anatomical abrreviations
ab, anal basal fulcra; af, anal fin; afr, anal fin rays; an, angular; asq, axial squamation; br, branchiostegal rays; cf, caudal fin; cl, cleithrum; cr, coronoid; cv, clavicle; dcb, dorsal caudal lobe basal fulcra; dcf, dorsal caudal lobe fringing fulcra; dcr, dorsal caudal lobe fin rays; df, dorsal fin; dfb, dorsal fin basal fulcra; dfr, dorsal fin rays; dn, dentary; dr; dorsal ridge scales; ds, dermosphenotic; ex, extrascapular; ff, fringing fulcra; fr, frontal; ju, jugal; la, lacrimal; ll, lateral line; lsq, lateral squamation; mnc, mandibular canal; mx, maxilla; na, nasal; op, opercular; pa, parietal; pcr, pectoral fin rays; pe, pelvic fin; pf, pectoral fin; pm, premaxilla; po, preopercular; por, sensory pores; pt, post-temporal; pvb, pelvic basal fulcra; pvr, pelvic fin rays; quj, quadratojugal; ra, radial; ro, rostral; rs, rostrum; scl, supracleithrum; sk, skull; sr, sclerotic ring; sn, sensory canal; so, subopercular; sq, squamation; th, teeth; vcb, ventral caudal lobe basal fulcra; vcr, ventral caudal lobe fin rays; vr, ventral ridge scales; vsq, ventral squamation.
Systematic palaeontology
Osteichthyes Huxley, 1880
Actinopterygii Cope, 1881
Family indet.
Genus TanyrhinichthysGottfried, 1987
Type and only species:
Tanyrhinichthys mcallisteriGottfried, 1987.
Holotype:
KUVP 83503, part and counterpart. Nearly complete, articulated fish with a poorly preserved skull and caudal fin, lacking dorsal and pectoral fins.
Type locality and horizon:
KUVP 83503 is from the Upper Pennsylvanian of north-central New Mexico, KBQ clay pit quarry locality, Bernalillo County, New Mexico (Gottfried, 1987).
Originally attributed to the Wild Cow Formation, the source formation for KUVP 83503 is now regarded as the Missourian Tinajas Member of the Atrasado Formation (Gottfried, 1987; Lucas et al., 2011; Williams & Lucas, 2013).
Additional material:
NMMNH P-51192 (part) and NMMNH P-51152 (counterpart), incomplete articulated fish including the skull but missing the anterior portion of the trunk; NMMNH P-70413 (part and counterpart), nearly complete articulated fish including the skull, but missing the caudal, median and pelvic fins; NMMNH P-70411, incomplete section of scales; NMMNH P-67687, impression of the body scales of the trunk; CM 30737, complete, articulated fish with a well-preserved skull, median fins, paired fins and caudal fin. All additional material is from the Missourian Tinajas Member of the Atrasado Formation at the KBQ.
Diagnosis (emended from Gottfried, 1987)
Elongate actinopterygian bearing a pronounced rostrum; rostrum composed of a prominent, pointed rostral, rostral contacted posteriorly by lengthened, paired frontals, pair of nasal bones surrounding the mid-posterior portion of the rostrum; rostrum base supported ventrally by a curved, strut-like premaxilla; frontals and parietals joined dorsal to the anteriormost edge of the orbit; rostral, frontal and parietals ornamented with crosswise ridges; single pair of extrascapulars with some crosswise ridges and concave posterior margins; post-temporals lacking ornamentation; mouth subterminal with small, curved, peg-like and sharply pointed teeth; dentary posteriorly deepened, with curved dorsal and ventral margins, and a pointed anterior margin; dentary ornamented with long, forward-curving ridges; maxilla ornamented with thin, sparse crosswise ridges; angular present; long, thin, anteriorly curved preopercular; tall cleithrum ornamented with thin, lengthwise ridges, with a rounded base and a pointed dorsal margin; rhombic scales bearing prominent dorsal pegs and ornamented with long, lengthwise ridges; dorsal ridge scales extending from the skull to the dorsal basal fulcra, grading from short, thick, rounded scales anteriorly into longer and more pointed scales posteriorly; ventral ridge scales extending from the base of the pectoral fin to the ventral caudal basal fulcra, grading from wide, rectangular, thick scales anteriorly into thinner, shorter and longer scales posteriorly; deepened scales in the lateral flank region; small, ventrally inserted paired fins; fringing fulcra absent on paired fins; offset median fins positioned far posteriorly with a larger, more anteriorly placed anal fin; fringing fulcra present on the anterior margin of the dorsal and caudal fin; basal fulcra present on the insertions of the pelvic fin, median fins and caudal fin; relatively small and shallowly cleft heterocercal caudal fin; dorsal and ventral lobe of caudal fin bearing large basal fulcra and smaller fringing fulcra; lepidotrichia of caudal fin closely packed, segmented and branching distally.
Description
Skull:
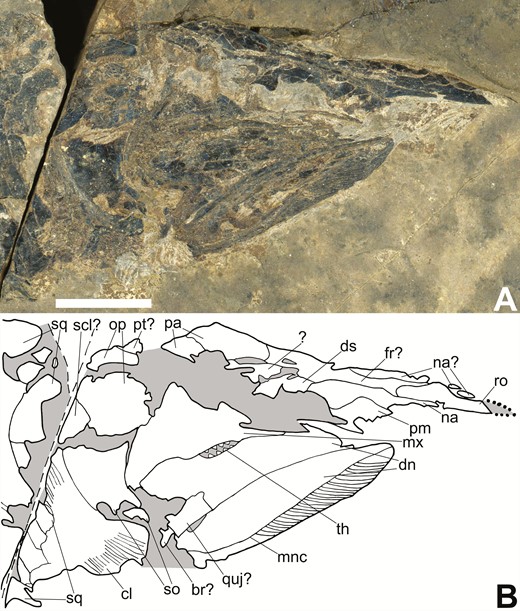
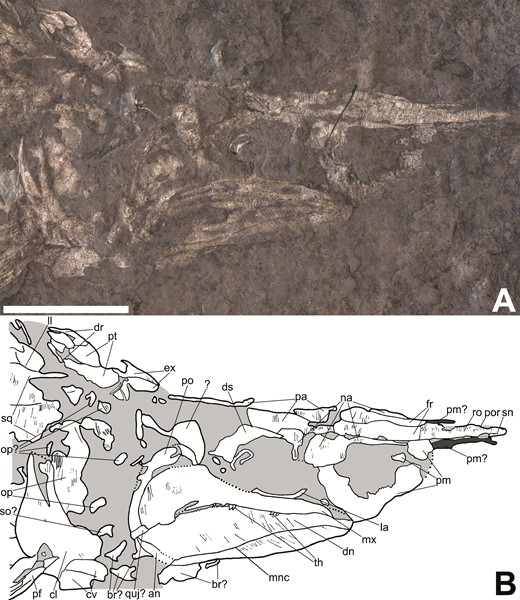
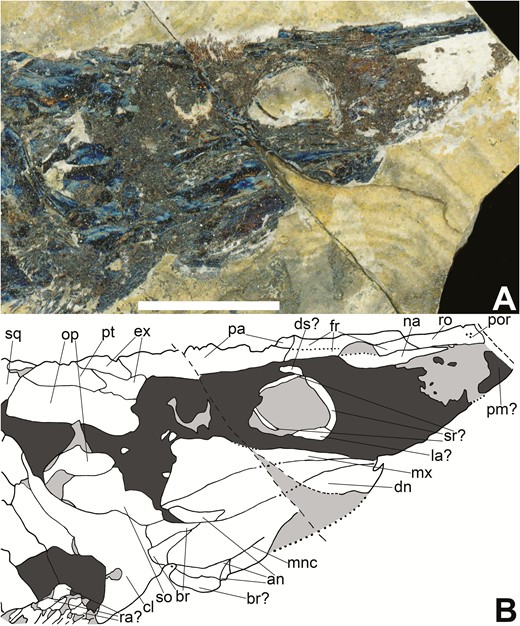
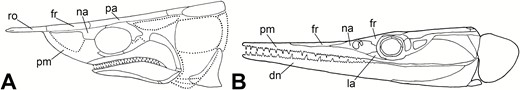
While the overall construction of the skull is as described by Gottfried (1987), the skull of the specimen used for this description (KUVP 83503) was severely crushed, rendering it difficult to adequately distinguish between individual bones and fractures (Gottfried, 1987). New specimens (NMMNH P-70413, CM 30737 and NMMNH P-51192) allow for a much more thorough description of the skull because they preserve many of the bones that could not be identified by Gottfried (1987). These include the dermosphenotic, frontals, nasals, extrascapulars, lacrimal, jugal, clavicle, premaxilla, branchiostegal rays, coronoids and angular. In addition, we re-examined the skull of KUVP 83503 (Fig. 3), and can provide identifications for several fragmentary bones based on information from the new material, including the nasals, parietals, dermosphenotic, premaxilla and frontals. Our identification of the elements in the skull of KUVP 83503 mostly align with those of Gottfried (1987) (Fig. 4), except that we identify a rectangular element in-between the dentaries as a possible quadratojugal, not a quadrate, and we did not observe a separate preopercular or supraorbital sensory canal.
Skull of the holotype of Tanyrhinichthys, KUVP 83503, preserved in lateral view (anterior is to the right). A, specimen photo. B, specimen drawing. Scale bar equals 1 cm.
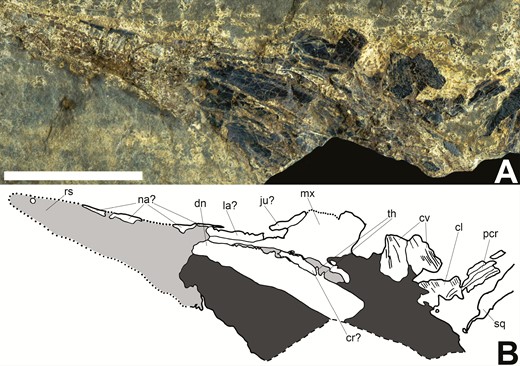
Most complete skull of Tanyrhinichthys, CM 30737, preserved in lateral view (anterior is to the right). A, specimen photo (colour inverted). B, specimen drawing. Scale bar equals 1cm.
The anterior portion of the skull of Tanyrhinichthys is extended into an elongate rostrum composed of multiple elements. CM 30737 (Figs 4, 7) bears the best preserved and only complete rostrum, although incomplete rostra are present in KUVP 83503 (Fig. 3), NMMNH P-70413 (Fig. 5) and NMMNH P-51192 (Fig. 6). The rostra of NMMNH P-70413, CM 30737 and KUVP 83503 are short, thick and pointed. These rostra are laterally flattened, giving them a thicker appearance than they would have had in life. The rostrum of NMMNH P-51192, preserved as an impression (‘rs’ in Fig. 6B), appears much longer and thinner than the rostra in CM 30737, KUVP 83503 and NMMNH P-70413 because it has been crushed dorsoventrally, not laterally. These specimens indicate that the rostrum of Tanyrhinichthys would have been relatively long and thin, most likely with a laterally broad dorsal surface.
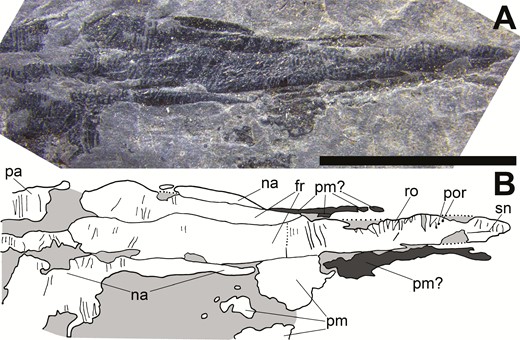
Skull of Tanyrhinichthys, NMMNH P-70413, preserved in lateral view (anterior is to the right). A, specimen photo. B, specimen drawing. Scale bar equals 1 cm.
Skull of Tanyrhinichthys, NMMNH P-51192, crushed ventrally (anterior is to the left). A, specimen photo. B, specimen drawing. Scale bar equals 1 cm.
The rostrum of Tanyrhinichthys, CM 30737 (anterior is to the right). A, specimen photo. B, specimen drawing. Scale bar equals 0.5 cm.
The most prominent element comprising the rostrum is a large, unpaired median rostral (‘ro’ in Figs 3B, 4B, 5B and 7B), which is bound laterally by an elongate pair of nasals and followed by a pair of elongate frontals (Fig. 7). The rostral is an elongated, roughly triangular bone that has a pointed anterior margin and a curved posterior margin. It extends past the nasals to form a roughly triangular point at the tip of the rostrum and is ornamented with parallel, crosswise ridges and small, tubercle-like protuberances. The rostral bears pores and a sensory canal anteriorly. While we only observe the canal in CM-30737 (‘sn’ in Figs 4B and 7B), rostral pores are visible in CM 30737 and NMMNH P-70413 (‘por’ in Figs 4B, 5B and 7B). These pores are equal in size, circular in shape and are shallowly placed at the margins of the bone. This canal and its associated pores are most likely a segment of the ethmoid commissure, a sensory canal that extends into the rostral bone of early actinopterygians (Gardiner, 1984). Fragmentary bone alongside the anterior portion of the rostral in CM 30737 and NMMNH P-70413 (‘pm?’, Figs 4B, 5B and 7B), suggests that the premaxillae may also contact the rostral ventrally, but the available specimens are not well-enough preserved to be certain.
The nasals are present in KUVP 83503 and NMMNH P-70413, but are best preserved in CM-30737 (‘na?’ and ‘na’ in Figs 3B, 4B, 5B, 6B and 7B). The nasals are a pair of elongate bones that can be divided into a long and thin anterior portion that contacts the premaxilla anteriorly and the frontals dorsally, and a broad, ventrally expanded posterior portion that contacts the frontals anterdorsally, the parietals posterodorsally and the dermosphenotic posteriorly. While the anterior portion lacks strong ornament, the posterior portion bears some crosswise ganoine ridges. There is a slight separation between the anterior and posterior portions of the right nasal in CM 30737 (most easily observed in Fig. 7A) that may represent a suture between a separate anterior and posterior nasal. While we cannot be certain without better preserved material, we interpret this separation as an area where bone is partially missing due to a break, not a suture.
The frontals are present but partially obscured in NMMNH P-70413, are potentially partially preserved in KUVP 83503 and are best preserved in CM 30737 (‘fr?’ and ‘fr’ in Figs 3B, 4B, 5B and 7B). They are elongated, roughly rectangular bones that form the posterior half of the lengthened rostrum and are ornamented with crosswise ganoine ridges. The frontals have rounded posterior margins where they contact the parietals and straight anterior margins where they contact the rostral. The frontals are bordered anteriorly by the rostral, laterally by the nasals and posteriorly by the parietals. There is a piece of bone in the centre of the rostrum of CM 30737 directly posterior to the rostral that we interpret as a partially broken anterior half of the right frontal. It is possible that this is a separate postrostral, but because there is no clear posterior margin that can be reliably distinguished from the thin cracks that run diagonally through the skull roof, we do not interpret this as a separate element. Additionally, there is no evidence for a separate postrostral in other specimens (KUVP 83503 and NMMNH P-70413) that preserve this section of the skull roof. However, we cannot be certain because of the crushed preservation of this region of the skull roof in CM 30737, KUVP 83503 and NMMNH P-70413. Better preserved skull roof material will be required to re-evaluate if a separate postrostral is present in Tanyrhinichthys.
The parietals (‘pa’ in Figs 3B, 4B, 5B and 7B) are preserved in KUVP 83503, NMMNH P-70413 and CM 30737. The parietals are elongate, rectangular bones that are ornamented with crosswise, parallel ridges. They are contacted anteriorly by the frontals, posteriorly by the extrascapulars, anterolaterally by the nasals, laterally by the dermosphenotics and posterolaterally by an unidentified element behind the orbit. The extrascapulars are not present in KUVP 83503, but are preserved in both NMMNH P-70413 and CM 30737 (‘ex’ in Figs 4B and 5B). They are short, roughly rectangular bones largely lacking ornament (besides a few thin ridges in CM 30737) that contact the parietals anteriorly, the post-temporals posteriorly and the opercular posteroventrally. The posterior margin of each extrascapular is concave where they contact the post-temporal. The post-temporals are potentially partially preserved in KUVP 83503 (‘pt?’ in Fig. 3B), but are more complete in NMMNH P-70413 and CM 30737 (‘pt’ in Figs 4B and 5B). They are long, unornamented and roughly oval-shaped bones that contact the extrascapulars anteriorly and the opercular ventrally.
The orbit is formed (moving clockwise from the top of the orbit) by the nasals, lacrimal, jugal and dermosphenotic. The dermosphenotic (‘ds’ in Figs 3B and 4B; ‘ds?’ in Fig. 5B) is present in KUVP 83503, CM 30737 and possibly NMMNH P-70413. It is best preserved in CM 30737, where it is a curved, roughly crescent-shaped bone forming the posterodorsal part of the orbit. The dermosphenotic has a broad ventral margin, a wide dorsal margin contacting the parietal and a pointed anterior margin contacting the nasal. A piece of bone that may represent the jugal (‘ju?’ in Fig. 6B) is preserved in NMMNH P-51192. The potential piece of the jugal in NMMNH 51192 is concave and curved, and is contacted posteriorly by the postorbital expansion of the maxilla. However, this piece and the surrounding elements in NMMNH 51192 are not preserved well enough to be certain of this identification. The lacrimal is a small, thin and concave bone, preserved in CM 30737 and possibly in NMMNH P-70413 and NMMNH P-51192 (‘la’ in Fig. 4B; ‘la?’ in Figs 5B and 6B). The lacrimal sits dorsal to the infraorbital expansion of the maxilla and anterior to the jugal, forming the ventral and anteroventral portion of the orbit. The premaxilla reaches the anteriormost part of the orbit in CM 30737, suggesting that it also contributed to the anterior margin of the orbit. The sclerotic ring may be preserved in NMMNH P-70413 (‘sr?’ in Fig. 5).
The region of the skull posterior to the eye and anterior to the opercular is not well preserved in any of the examined specimens. In CM 30737 there is a large piece of bone (‘?’ in Fig. 4B) in the area of the skull posterior to the dermosphenotic and anterior to the opercular that appears to be a single element. This element is anteriorly broad and curved and has a long, thin projection extending posteriorly. There is a similar piece of bone located directly posterior to the dermosphenotic in KUVP 83503 (‘?’ in Fig. 3B), which is also poorly preserved. These pieces are not preserved well enough to determine if there is a single element (a fused dermopterotic) or two elements (a separate intertemporal and supratemporal) in this region of the skull. Therefore, we do not attempt an identification. We also cannot determine if there are separate suborbital bones.
The premaxilla is preserved in CM 30737, KUVP 83503 and NMMNH P-70413 (‘pm’ in Figs 3B, 4B and 7B). It is best preserved in CM 30737, where it seems to have a broad posterior margin that extends dorsally from the anteriormost tip of the maxilla to the most ventral point of the nasals. It also has a curved, strut-like section that extends anterodorsally, contacting the anterior section of the frontals and the posterior part of the rostral at the midpoint of the rostrum. In CM 30737 there are long pieces of bone lateral to the rostral (‘pm?’ in Figs 4B and 7B) that may also represent the premaxilla. Because these are disarticulated and not well preserved, we cannot determine if these represent the anterior extent of the premaxilla or if this is disarticulated bone that was fossilized next to the rostral. There is also a large gap in the region ventral to the rostrum in both NMMNH P-70413 and CM 30737 that may represent the actual interior border of the premaxilla or the product of decomposition after death. Because the premaxilla is not completely preserved in KUVP 83503, NMMNH P-70413 or CM 30737, its exact shape cannot be determined.
Opercular series:
The opercular series of Tanyrhinichthys is largely as reconstructed by Gottfried (1987). The preopercular is only preserved in CM 30737 (‘po’ in Fig. 4B). Although Gottfried (1987) (Fig. 4) originally identified a possible preopercular in KUVP 83503, this specimen is not preserved well enough to identify a separate preopercular. The preopercular is a long, thin, crescent-shaped bone that broadens into a circular expansion at its anteriormost point as it curves over the maxilla at a relatively shallow angle. The posterior margin of the preopercular is straight, while the anterior margin of the preopercular is broader and rounded. The preopercular contacts the unidentified element posterior to the dermosphenotic anterodorsally, the maxilla anteriorly and the possible quadratojugal ventrally. The subopercular is most complete in KUVP 83503, is present but is not well preserved in NMMNH P-70413 and is possibly present as a fragment in CM 30737 (‘so’ and ‘so?’ in Figs 3B, 4B and 5B). The subopercular is a tall, anteriorly concave bone with a broad dorsal margin and a narrower ventral margin that lacks ornament. The subopercular is contacted posteriorly by the cleithrum, dorsally by the opercular and ventrally by the branchiostegal rays. The opercular is broken in KUVP 83503 and is present but not better preserved in CM 30737 or NMMNH P-70413 (‘op?’ and ‘op’ in Figs 3B, 4B and 5B). The opercular is a broad, roughly circular bone that is lightly ornamented with crosswise ridges. It is contacted ventrally by the suboperculum, posterodorsally by the post-temporal, anterodorsally by the extrascapular and posteroventrally by the supracleithrum.
Gulars and branchiostegals:
The gulars are not preserved in any of the examined material. Pieces of the branchiostegal rays are preserved in KUVP 83503, CM 30737 and NMMNH P-70413 (‘br’ and ‘br?’ in Figs 3B, 4B and 5B). The branchiostegal rays are represented by disarticulated fragments in KUVP 83503 and CM 30737, which provide little information on their number and shape. However, the two articulated branchiostegal rays (and a third disarticulated element that is likely a branchiostegal ray) in NMMNH P-70413 show that these elements extended dorsally around the posterior margin of the dentary to the ventral margin of the subopercular, contacting the cleithrum posteriorly. Additional material that better preserves the ventral aspect of the skull will be required for a detailed description of the gulars and the shape and number of branchiostegal rays.
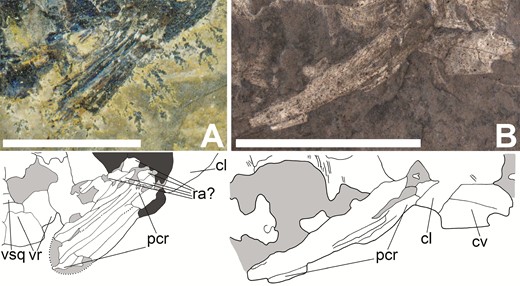
Shoulder girdle:
The shoulder girdle is largely as described by Gottfried (1987). The cleithrum is preserved in KUVP 83503, CM 30737, NMMNH P-70413 and NMMNH P-51192 (‘cl’ in Figs 3B, 4B, 5B, 6B and 8). The cleithrum is a tall, crescent-shaped bone that is broad and slightly rounded at its base with a round, pointed dorsal margin. Along its anterior margin (from dorsal to ventral) the cleithrum is contacted by the opercular, subopercular, branchiostegal rays and clavicles. The cleithrum is ornamented by thin, curved, lengthwise ganoine ridges. A crescent-shaped piece of bone above the cleithrum in KUVP 83503 (‘scl?’ in Fig. 3B) may represent part of a supracleithrum. However, this element and the region of the skull around it are broken and incomplete. Therefore, we cannot be certain of this identification, or describe the shape or size of the supracleithrum in detail. The clavicles (‘cv’ in Figs 4B, 6b and 8B) are preserved in CM 30737 and NMMNH P-51192. In CM 30737 they are attached to the anterior part of the ventral margin of the cleithrum. While their anterior margins are not well-preserved, the clavicles have rounded, convex posterior margins and narrow anteriorly. The clavicles in NMMNH P-51192 are ornamented with thin, curved and lengthwise ganoine ridges, while the clavicles in CM 30737 do not have ornament. This may be the result of differences in preservation or even intraspecific variation in bone ornamentation. More specimens preserving the clavicles will be required to evaluate this variation fully.
Pectoral fins of Tanyrhinichthys (anterior is to the right). A, NMMNH P-70413 pectoral fin. B, CM 30737 pectoral fin (colour inverted in specimen photo). Scale bars equal 1 cm.
Jaws and dentition:
The jaws and dentition of Tanyrhinichthys are largely as described by Gottfried (1987). The maxilla is preserved in KUVP 83503, CM 30737, NMMNH P-70413 and NMMNH P-51192 (‘mx’ in Figs 3B, 4B, 5B and 6B). It has a broad, rounded postorbital expansion and a long, thin suborbital process. The dorsal margin of the postorbital expansion of the maxilla is curved, and the dorsal margin of its suborbital process is concave. The ventral margin of the maxilla is also deeply concave. The maxilla contacts the preopercular posteriorly and dorsally, the potential quadratojugal posteriorly, the dentary ventrally and the lacrimal dorsally. It is ornamented with thin, parallel ridges. The dentary is preserved in CM 30737, NMMNH P-70413, NMMNH P-51192 and KUVP 83503 (‘dn’ in Figs 3B, 4B, 5B and 6B). It is a stout, posteriorly deepened bone with curved dorsal and ventral margins and is ornamented with long, forward-curving ganoine ridges. A prominent mandibular canal is preserved in the dentaries of KUVP 83503, NMMNH P-70413 and CM 30737 (‘mnc’ in Figs 3B, 4B and 5B). The mandibular canal originates in the ventral part of the angular. It is initially straight as it extends into the dentary, but approximately midway through the dentary it curves dorsally, continuing anteriorly to the anterior margin of the dentary. A disarticulated, ovoid element that is likely a coronoid is preserved in NMMNH P-51192 (‘cr?’ in Fig. 6B). This may also be a piece of the prearticular, the lower jaw is not well-enough preserved in this specimen to make a certain identification. There is a small, concave, curved angular contacting the posterior margin of the dentary (‘an’ in Figs 4B and 5B). The angular is preserved in CM 30737 and NMMNH P-70413 and can be distinguished from the dentary by its lack of ornamentation. We did not observe any evidence for the presence of a separate surangular, but the posterior dorsal region of the lower jaw is not well preserved in any of the examined material. Therefore, we cannot definitively determine if Tanyrhinichthys possessed two infradentaries. KUVP 83503 and CM 30737 both preserve small, roughly rectangular elements contacting the posterodorsal margin of the dentary, which may represent quadratojugals (‘quj?’ in Figs 3B and 4B). We do not attempt a certain identification because this region of the skull is not well preserved in either specimen. Tanyrhinichthys has a strongly subterminal mouth with small, peg-like, curved and sharply pointed teeth with acrodin caps that are preserved in KUVP 83503, CM 30737 and NMMNH P-51192 (‘th’ in Figs 3B, 4B and 6B). These teeth are in one row with little variation in shape or size between them.
Paired fins:
The pelvic fin is poorly preserved in KUVP 83503 (‘pe’ in Fig. 1) and the pectoral fin is completely absent. Therefore, the pectoral and pelvic fins in the new material provide a wealth of novel morphological information, particularly on their size and shape (Figs 8, 9). Partial pectoral fins are present in NMMNH P-70413 (Fig. 8A), CM 30737 (‘pf’ in Fig. 2B; Fig. 8B) and NMMNH P-51192 (‘pcr’ in Fig. 6B). The most complete pectoral fin is present in NMMNH P-70413, showing that it is small, with a 45-degree insertion into the shoulder girdle. The lepidotrichia in the pectoral fins (‘pcr’ in Fig. 8) are large, thick, cylindrical, unjointed, unbranching and densely packed. NMMNH P-70413 preserves fragmentary elements proximal to the lepidotrichia that may be pieces of the radials (‘ra?’ in Fig. 8A). These elements are not preserved well enough for a certain identification. There is no evidence of fringing fulcra preceding the pectoral fin. Because none of the specimens bear a complete pectoral fin, its exact shape is not known. The available material suggests that it is a short fin, narrow at its base, that broadens into a rounded distal margin.
Pelvic fin of Tanyrhinichthys, CM 30737 (anterior is to the right). A, specimen photo (colour inverted). B, specimen drawing. Scale bar equals 1 cm.
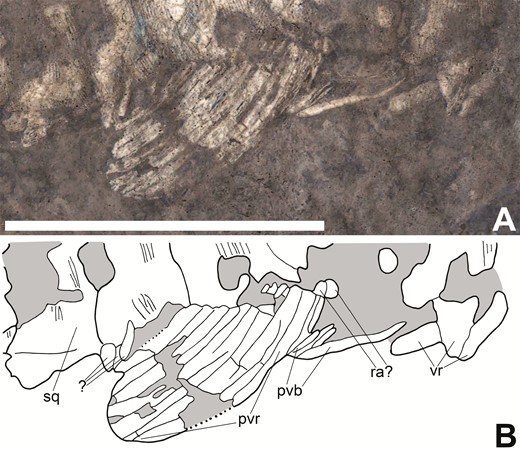
The pelvic fin is represented in KUVP 83503 by a rounded patch of fin rays located approximately halfway along the ventral margin of the body (‘pe’ in Fig. 1). The pelvic fin is well preserved in CM 30737 (‘pe’ in Fig 2; Fig 9), showing that it is a small, rounded fin with a broad base. The lepidotrichia are of medium thickness, unbranched, lightly segmented and closely packed (‘pvr’ in Fig. 9B). Small, round elements dorsal to the lepidotrichia may represent radials (‘ra?’ in Fig. 9B). However, this identification is not certain because the insertion of the fin is not well preserved. Small, thin and pointed pelvic basal fulcra are located directly anterior to the pelvic fin, with a longer, thinner pelvic basal fulcrum located anterior to these (‘pvb’ in Fig. 9B). Anterior to these fulcra are ventral ridge scales (‘vr’ in Fig. 9B). We were unable to identify several small, teardrop-shaped elements located posterior to the pelvic fin, because it is not clear if these are separate from the ventral ridge scales posterior to the pelvic fin or pieces of bone from the fin that were moved to their current position post-fossilization (‘?’ in Fig. 9B).
Median fins:
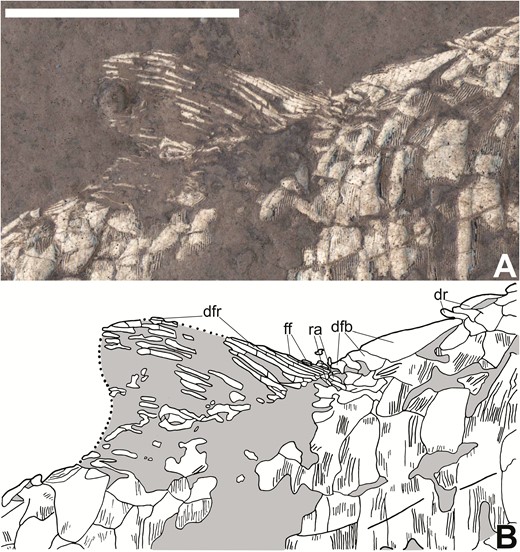
The dorsal fin is absent in the holotype and is known only from a partially complete fin in CM 30737 (‘df’ in Fig 2; Fig 10). This fin is small and rounded, has its peak in its posterior half and is placed in the posterior part of the dorsal margin of the body. The lepidotrichia are lightly segmented, unbranching, small, thin and closely packed (‘dfr’ in Fig. 10B). Although much of the attachment of the dorsal fin to the body is not preserved, several small radials are present ventral to the anterior insertion (‘ra’ in Fig. 10B). Several small, thin, pointed elements at the anterior insertion of the fin, formed from expanded terminal segments of the leading lepidotrichia, are likely fringing fulcra (‘ff’ in Fig. 10B). Directly anterior to the fringing fulcra are three short, small and thick dorsal basal fulcra, which are followed by a single, much larger dorsal basal fulcrum (‘dfb’ in Fig. 10B). Dorsal ridge scales sit directly anterior to the large dorsal basal fulcrum (‘dr’ in Fig. 10B).
Dorsal fin of Tanyrhinichthys, CM 30737 (anterior is to the right). A, specimen photo (colour inverted). B, specimen drawing. Scale bar equals 1 cm.
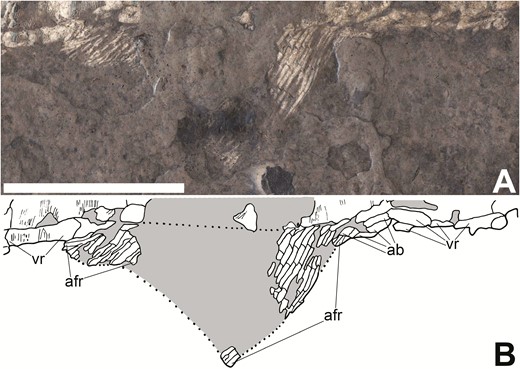
The anterior third of the anal fin is present in KUVP 83503, showing that it was positioned farther posteriorly along the body than in most other early actinopterygians (‘af’ in Fig. 1B). CM 30737 exhibits a partial but more complete anal fin that provides more information on its size and shape (‘af’ in Fig 2B; Fig 11). The anal fin of CM 30737 consists of three patches of fin rays. These patches represent the anterior insertion and a portion of the anterior margin, a disarticulated patch that may be from the distal peak of the fin and the posterior insertion of the fin and the area surrounding it. The anal fin is placed anterior to the dorsal fin and has a considerably broader base. The posterior portion of the anal fin is short and rounded, while the anterior portion of the fin is longer and more triangular. While the anterior margin of the anal fin is not complete, the articulated patch of lepidotrichia from the anterior insertion is taller than the posterior margin, indicating that the peak was in the anterior half. The lepidotrichia in the anterior portion of the anal fin are densely packed, regularly segmented and do not branch (‘afr’ in Fig. 11B). The lepidotrichia in the posterior part of the anal fin are smaller, thinner, lightly segmented and shallowly branched distally (‘afr’ in Fig. 11B). The anal fin is preceded by at least two pairs of short, thick basal fulcra (‘ab’ in Fig. 11B) and paired ventral ridge scales (‘vr’ in Fig. 11B). We do not observe fringing fulcra, but the anterior margins of this fin in CM 30737 and KUVP 83503 are not preserved well enough to determine if these elements were present with certainty.
Anal fin of Tanyrhinichthys, CM 30737 (anterior is to the right). A, specimen photo (colour inverted). B, specimen drawing. Scale bar equals 1 cm.
Tail and caudal fin:
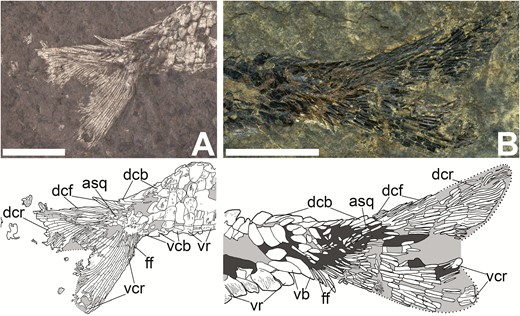
While the caudal fin is present in KUVP 83503 (‘cf’ in Fig. 1), the distal regions of the dorsal and ventral lobes and the median cleft of the fin are poorly preserved. Better preserved caudal fins are present in CM 30737 (‘cf’ in Fig 2; Fig 12A) and NMMNH P-51192 (Fig. 12B). The caudal fin of Tanyrhinichthys is relatively small and heterocercal, with a long and roughly triangular dorsal lobe with a rounded margin. The ventral lobe is shorter and thicker than the dorsal lobe, and also has a rounded margin. The area between the dorsal and ventral lobes is not well preserved in any of the specimens, but the available material indicates that the caudal fin had a relatively shallow median cleft. The lepidotrichia in the ventral lobe of the caudal fin (‘vcr’ in Fig. 12A, B) are thin, segmented, closely packed and branch distally. The lepidotrichia of the dorsal lobe (‘dcr’ in Fig. 12A, B) are also segmented, closely packed and branching distally, but are thicker. The lepidotrichia in the ventral lobe of NMMNH P-51192 (‘vcr’ in Fig. 12B) are much thicker than those in the ventral lobe of CM 30737 (‘vcr’ in Fig. 12A).
Caudal fin of Tanyrhinichthys. A, CM 30737 (anterior is to the right, colour inverted in specimen photo). B, NMMNH P-51192 (anterior is to the left). Scale bars equal 1 cm.
The posterior portion of the dorsal surface of the caudal peduncle is covered by a series of large triangular basal fulcra (‘dcb’ in Fig. 12). The caudal basal fulcra each have a deeply concave dorsal margin that fits around the long, pointed posterior margin of the preceding fulcrum. A series of fringing fulcra that cover the dorsal margin of the caudal fin (‘dcf’ in Fig. 12) sit directly posterior to the caudal basal fulcra. These fringing fulcra become progressively longer and thinner posteriorly and have pointed apices. The axial squamation is preserved in both CM 30737 and NMMNH P-51192 (‘asq’ in Fig. 12). In these specimens, the scales on the caudal peduncle grade into smaller, more elongate, thinner and more pointed scales on the dorsal lobe. A shorter, less prominent series of two to three pairs of basal fulcra are present on the ventral lobe of the caudal fin of CM 30737 and NMMNH P-51192 (‘vcb’ in Fig. 12). We also observed some fringing fulcra posterior to the basal fulcra on the ventral lobe of the caudal fins of CM 30737 and NMMNH P-51192 (‘ff’ in Fig. 12).
Squamation:
The squamation is well preserved in KUVP 83503 (Fig. 1), CM 30737 (Fig. 2) and NMMNH P-70413 and is present to some degree in every known specimen of Tanyrhinichthys. The squamation is largely as described by Gottfried (1987): the scales of Tanyrhinichthys are rhombic, ganoine-covered and possess peg-and-socket articulations. The pegs are thick, triangular, short and pointed, located on the posterodorsal margin of the scale. These features are typical of the scales of early actinopterygians (Moy-Thomas, 1971). The scales are ornamented with vertical, roughly parallel ridges, which often extend from the dorsal to the ventral external margin (Fig. 2). The scales in the lateral flank region are deeper than they are wide, and the scales in the anterior portion of the body are larger and deeper than those located more posteriorly. The scales become smaller and more rhomboidal on the caudal peduncle, different in shape and size from the scales of the rest of the body. The transition from body to caudal peduncle scale rows was not preserved in KUVP 83503, but CM 30737 and NMMNH P-51192 have well-preserved caudal peduncles that provide this information. The scales smoothly transition into smaller versions of the large and deepened body scales and become smaller and more rhomboidal towards the caudal fin.
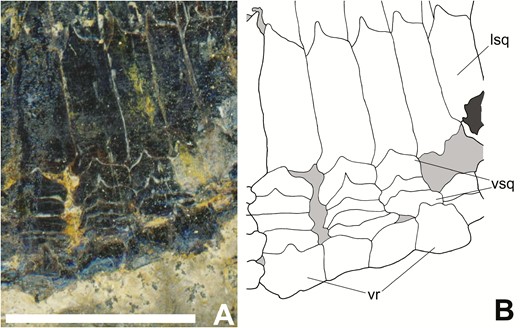
Dorsal ridge scales (‘dr’ in Figs 1 and 2), present in KUVP 83503 and CM 30737, run from the base of the skull to the basal fulcra on the dorsal fin. They are short, thick and rounded anteriorly, becoming longer, thinner and more pointed posteriorly. The ventral squamation is best preserved in NMMNH P-70413 (Fig. 13) and is fragmentarily preserved in CM 30737 and KUVP 83503. Only NMMNH P-70413 preserves a series of three to six rows of squat, trapezoidal scales (‘vsq’ in Fig. 13B) running along the ventral surface of the body, extending from the base of the pectoral fin to the caudal peduncle. These scales are shorter than the scales covering the trunk and have smaller, less prominent dorsal pegs. Ventral to these in NMMNH P-70413 is a row of ventral ridge scales (‘vr’ in Figs 9 and 11–13) that are distinguishable from the ventral squamation in being larger and thicker than the scale rows above them. The ventral ridge scales extend from directly posterior to the pectoral fin to the ventral caudal basal fulcra, but are interrupted by the pelvic and anal fins, with their respective basal fulcra. Anteriorly, these scales are squat, wide, thick and roughly rectangular in shape. Although a section of them appears to be missing from the mid-posterior region of the body of NMMNH P-70413, they become thinner, shorter and longer as they approach the caudal peduncle.
Squamation along the ventral margin of the anterior lateral flank of Tanyrhinichthys, NMMNH P-70413. A, specimen photo (anterior is to the right). B, specimen drawing. Scale bar equals 1 cm.
DISCUSSION
The palaeoecology of Tanyrhinichthys
Previous workers have argued that Tanyrhinichthys was ecomorphologically similar to ram-feeding, esocid-like predators, based on the original reconstruction (Gottfried, 1987, fig. 6A; Moyle & Cech, 2003; Williams & Lucas, 2013). Ram-feeding fishes, including pikes (Esocidae), are often lie-in-wait predators with fusiform bodies, broad, homocercal caudal fins, posteriorly placed dorsal and anal fins with similar forms and positions, and lengthened, terminal mouths with large, conical teeth (Webb, 1984b; Moyle & Cech, 2003; Porter & Motta, 2004). These specialized features allow esociforms to perform sudden, high-velocity lunges at prey; the mirrored median fins, deeply forked homocercal tail and elongated, streamlined body-form serving to maximize thrust while minimizing drag (Webb & Skadsen, 1980; Webb, 1984a; Moyle & Cech, 2003; Porter & Motta, 2004). Our reconstruction of Tanyrhinichthys (Fig. 14) shows a body-form distinct from esociforms, including displaced median fins unequal in form and a highly heterocercal caudal fin, ruling out an ability to generate equivalent bursts of forward motion. Furthermore, the esociform style of ram feeding requires a terminal mouth to capture prey head-on (Webb, 1984b; Moyle & Cech, 2003; Porter & Motta, 2004). The mouth of Tanyrhinichthys is subterminal. Thus, an ‘ambush predator’ ecology can now be ruled out for Tanyrhinichthys.
A, reconstruction of Tanyrhinichthys, based primarily on CM 30737. B, life restoration. Scale bar equals 1 cm.
We reinterpret Tanyrhinichthys as a benthic-cruising predator, likely similar in general feeding ecology to sturgeon (Acipenseridae) (Billard & Lecointre, 2000). This interpretation is supported by features shared between Tanyrhinichthys and sturgeon, including a heterocercal tail with a long dorsal lobe, an elongate snout bearing a sensory canal and ventrally inserted paired fins (Bemis et al., 1997; Miller, 2004; Vecsei & Peterson, 2004; Peterson et al., 2007; Hilton et al., 2011). Additionally, a large fossa in the skull of Tanyrhinichthys, formed by the premaxilla and rostrum, may have contained soft tissue with additional sensory organs, such as electroreceptors. However, better preserved material is required to evaluate this possibility. These shared features have been documented as facilitating a bottom-cruising predatory lifestyle in sturgeon and, therefore, most likely served a similar purpose in Tanyrhinichthys. For instance, the inequilobate tail and elongate anal fin in Tanyrhinichthys likely would have assisted with both descent to the bottom and rapid movement off the substrate. The ventrally placed paired fins likely would have helped with station holding, as in modern sturgeon (Adams et al., 1999; Liao & Lauder, 2000). The sensory apparatus on the rostrum of Tanyrhinichthys, together with its subterminal mouth, suggest that it searched for food in a manner similar to modern sturgeon: swimming along the bottom and using the sensory organs associated with its rostrum to detect prey hidden in the substrate (Harkness & Dymond, 1961). This comparison is limited by the fact that sturgeon bear soft-tissue rostral sensory organs (including chemosensory barbels and epithelial electrosensory ampullary organs) (Jørgensen, 1980; Hilton et al., 2011). While it is possible that Tanyrhinichthys possessed similar electrosensory or chemosensory organs, it is unlikely that the restrictions of the fossil record will allow for this to be determined.
Differences in jaw morphology also limit the inferred convergence between Tanyrhinichthys and sturgeon. While sturgeon have a highly specialized, protractible mouth that sucks in prey by rapid extension, Tanyrhinichthys has an upper jaw (maxilla) that is tightly fused to the rest of its skull, as in most other Palaeozoic actinopterygians (Schaeffer & Rosen, 1961; Vecsei & Peterson, 2004; Peterson et al., 2007). The fusion of the maxilla to the preopercular and infraorbital bones restricted Tanyrhinichthys and other Palaeozoic actinopterygians to biting and seizing prey (Schaeffer & Rosen, 1961). Therefore, despite the apparent convergence between them, Tanyrhinichthys and sturgeon are distinct in mode of prey capture. Stomach contents have not been recovered, but the small, sharp, curved and peg-like teeth, and relatively small gape of Tanyrhinichthys indicate that it fed upon small crustaceans, insects and soft-bodied organisms (Williams & Lucas, 2013).
Comparisons to other Palaeozoic taxa
As noted by Gottfried (1987), Tanyrhinichthys possesses general characteristics of early actinopterygians traditionally assigned to the likely para- or polyphyletic taxonomic group for Palaeozoic species, ‘paleonisciformes’ (Sallan, 2014). This includes rhombic, ganoine-covered scales with peg-and-socket articulations, a strongly heterocercal caudal fin and a maxilla with a pronounced, rounded postorbital expansion and narrow suborbital expansion (Moy-Thomas, 1971; Sallan, 2014). Phylogenetic analysis of Tanyrhinichthys is difficult because the available material is flattened and thus lacks many of the internal features that have proven the most informative for determining phylogenetic structure in prior analyses (Sallan, 2014; Giles et al., 2015; Pradel et al., 2016; Argyriou et al., 2018; Latimer & Giles, 2018; Wilson et al., 2018; Coates & Tietjen, 2019; Figueroa et al., 2019). Also, the relationships of Permo-Carboniferous actinopterygians are poorly defined and relatively under-examined; most prior analyses involved either Mississippian and Late Permian taxa and/or focused on a subset of Late Palaeozoic species belonging to one region or family (Lowney, 1980; Dietze, 2000; Sallan, 2014; Elliott, 2015; Elliott, 2018). Lastly, most other actinopterygians of the same age from North America have only been briefly described. Thus, phylogenetic placement of Tanyrhinichthys will require a detailed examination of many Pennsylvanian and Early Permian taxa at KBQ and elsewhere that is outside of the scope of the present work. Therefore, we do not attempt to determine the placement of Tanyrhinichthys within the ‘paleonisciformes’. Instead, we compare Tanyrhinichthys to other long-rostrumed Palaeozoic actinopterygians to determine the possibility of shared evolutionary pathways or close relationships and to examine the early evolutionary history of elongate rostra in ray-finned fishes.
The Palaeozoic Trawdenia planti (Coates & Tietjen, 2019) and Illinichthys cozarti (Schultze & Bardack, 1987) both possess prominent snouts that extend beyond the gape (Schultze & Bardack, 1987; Coates, 1999; Coates & Tietjen, 2019). These are formed primarily from a bulbous, inflated rostral bone, with contributions from the nasal and premaxilla (Schultze & Bardack, 1987; Coates, 1999; pers. observ.). Although these structures are not as elongate as the snout of Tanyrhinichthys, they may represent precursor states. Most of the other features of these genera are common to a broader range of Permo-Carboniferous actinopterygians, thus the degree of relatedness is otherwise difficult to determine, as above.
While the Late Devonian Tegeolepis and Tanyrhinichthys both bear elongate rostra, there are several morphological distinctions between these fishes. Principally, unlike Tanyrhinichthys, the rostrum of Tegeolepis is composed entirely of an inflated, pointed rostral bone (Dunkle & Schaeffer, 1973). This is distinct from the rostrum of Tanyrhinichthys, which also is composed of a lengthened median rostral but has contributions from the paired frontals, nasals, premaxillae and parietals. Additionally, unlike Tanyrhinichthys, the pectoral fins of Tegeolepis contain deeply branched fin rays and the median fins lack fringing and basal fulcra (Gardiner, 1963).
Differences in body size, body shape and dentition between Tanyrhinichthys and Tegeolepis indicate divergent ecologies. While Tanyrhinichthys is a relatively small fish (approximately 15 cm in total length), Tegeolepis is huge for a Palaeozoic actinopterygian, between 60 and 100 cm in total length (Dunkle & Schaeffer, 1973). Additionally, Tegeolepis has two series of teeth (marginal and internal) that include large, recurved laniaries (Dunkle & Schaeffer, 1973). This differs considerably from the single set of small, peg-like teeth of Tanyrhinichthys. The body form and fin positions of Tegeolepis are decidedly more esociform-like, including the presence of small, mirrored median fins near the tail (Dunkle & Schaeffer, 1973). While Tanyrhinichthys was most likely a bottom-feeder that inhabited an estuarine environment, Tegeolepis has been interpreted as a fast-swimming, pelagic predator that inhabited a marine environment (Gardiner, 1963; Dunkle & Schaeffer, 1973; Long, 2011). Therefore, the superficially similar rostral forms of these fishes appear to have evolved independently and for completely divergent uses.
The Saurichthyiformes were an extremely successful group of long-rostrumed fishes whose earliest recorded representative, Eosaurichthys, is known from the latest Permian (Changhsingian) of Zhejiang, China (Liu & Wei, 1988; Argyriou et al., 2018). Saurichthyiformes like Eosaurichthys are distinct from Tanyrhinichthys in general body form. While Saurichthyiformes have a homocercal tail and mirrored median fins, Tanyrhinichthys has a heterocercal tail and median fins that are not mirrored (see: Kogan & Romano, 2016 and references therein). Additionally, while the rostrum of Tanyrhinichthys is built primarily from lengthened dermal bones of the skull roof (frontals) and dermal bones associated with the ethmoid region (rostral, nasals and premaxillae), the rostrum of Eosaurichthys is formed primarily from lengthened elements that comprise the jaw margin (premaxillae and dentaries) (Liu & Wei, 1988; Kogan & Romano, 2016). While dermal skull roof bones (frontals) and dermal bones associated with the ethmoid region (nasals) are also lengthened in Eosaurichthys, its rostrum is distinct in overall form from that of Tanyrhinichthys (Liu & Wei, 1988; Kogan & Romano, 2016). Also, Tanyrhinichthys has an extended snout-like structure while Eosaurichthys has a lengthened mouth (Fig. 15). The distinctions between the elongate rostra of these fishes are most likely due to the difference in their inferred ecologies. Unlike the inferred bottom-roving feeding strategy of Tanyrhinichthys, the needlefish or barracuda-like forms of Eosaurichthys and other Saurichthyiformes indicate that they were likely pelagic, ram-feeding ambush predators (Kogan et al., 2015). The elongate jaws of Eosaurichthys and other Saurichthyiformes appear to be convergent on extant taxa (notably pike, needlefish, gar and barracuda) whose lengthened jaws are well suited for high-velocity closure to capture fast-swimming fishes (Porter & Motta, 2004; Kogan et al., 2015; Kogan & Romano, 2016). Thus, the distinction in the form of the elongate rostra of these fishes, together with other morphological differences, are likely due to Eosaurichthys being more ecologically similar to pike and possibly Tegeolepis than Tanyrhinichthys.
Comparison of the two broad structural forms of elongate rostra in Palaeozoic actinopterygians. A, Tanyrhinichthys, which bears an elongate rostrum that is a lengthened snout-like structure above the mouth. B, a representative saurichthyiform (Saurichthys madagascariensis Piveteau, 1945), which bears an elongate rostrum that is a lengthened mouth (after Kogan & Romano, 2016, fig. 11B).
While some morphological distinctions between Tanyrhinichthys and Eosaurichthys appear to be due to divergent ecologies, a recent study of the internal cranial anatomy of Saurichthys sp. found that it is likely part of a clade that is an immediate sister-group to crown actinopterygians, while Tanyrhinichthys is difficult to distinguish from the mass of Carboniferous forms that fall lower down along the stem (but see discussion of phylogenetic status above) (Argyriou et al., 2018). Although this topology is weakly supported, phylogenetic study has consistently placed Saurichthyiformes with Triassic taxa (see: Argyriou et al., 2018 and references therein). This (along with the considerable temporal gap between these taxa) indicates that Eosaurichthys is part of a younger lineage of ray-finned fishes than Tanyrhinichthys. Therefore, the available data indicate that elongate rostra evolved independently in Tanyrhinichthys and Eosaurichthys.
Phanerorhynchus is the best-known, long-rostrumed actinopterygian that is contemporaneous with Tanyrhinichthys (Gill, 1923; Fig. 16). Our comparison is based on the original description (Gill, 1923), examination of latex peels (P.34421–2 and P. 50023–4, NHM) taken from the holotype (L. 8585), microCT scans of L. 8585 (provided by Matt Friedman), and the reconstruction and description of Phanerorhynchus from Gardiner (1967), which is unfortunately highly idealized (pers. observ.). Like Tanyrhinichthys, Phanerorhynchus superficially resembles sturgeon, as noted by D. M. S Watson in Gill (1923) and Gardiner (1967). Both Tanyrhinichthys and Phanerorhynchus possess a pronounced rostrum, posteriorly placed median fins and a subterminal mouth (Gill, 1923; Gardiner, 1967; Miller, 2004; Vecsei & Peterson, 2004; Peterson et al., 2007). These shared features suggest that, like Tanyrhinichthys, Phanerorhynchus was a small, bottom-cruising predator (Gill, 1923; Gardiner, 1967).
A, photograph of a latex peel (P. 34421–2) of the holotype Phanerorhynchus, scale bar equals 1 cm. B, specimen drawing of the holotype of Phanerorhynchus (L. 8585), after Gill (1923). Anterior is to the left.
Much of the skull of the lone specimen of Phanerorhynchus, particularly its rostrum and skull roof, is not well-enough preserved for an in-depth comparison to Tanyrhinichthys (Gill, 1923; ‘sk’ in Fig. 16), with the exception of the jaws, dermal cheek bones and orbit. The rostrum of Phanerorhynchus is thicker and more conical than that of Tanyrhinichthys (Gill, 1923), presenting a marked difference despite the undefined contributions of the fused dermal snout bones in Phanerorhynchus. Additionally, the frontals of Phanerorhynchus make up a much larger portion of the skull roof and are larger relative to the parietals than those of Tanyrhinichthys.
In many respects the skull of Phanerorhynchus is distinct from Tanyrhinichthys and is more similar to the haplolepids, a group of Carboniferous actinopterygians known from both the UK and North America (Lowney, 1980; Elliott, 2015, 2018). The skull bones of Phanerorhynchus were ornamented with thick, concentric ridges and tubercles, similar to haplolepids yet distinct from the lightly ornamented or unornamented skull bones of Tanyrhinichthys (Gill, 1923; Westoll, 1944; Lowney, 1980). The maxilla of Phanerorhynchus is broad and expanded posteriorly, relative to what is typical of other ‘paleoniscoids’ (Gill, 1923; Gardiner, 1967), similar to Haplolepidae, excluding Microhaplolepis (Westoll, 1944; Lowney, 1980). In contrast, the maxilla of Tanyrhinichthys is narrower and more boomerang-shaped, and thus more typical of the ‘paleoniscoids’. Additionally, the preopercular of Phanerorhynchus is wider and much broader dorsally than the preopercular of Tanyrhinichthys (Gill, 1923). Other distinctions between the skulls of these fishes lie in the construction of the orbit and the surrounding bones. Unlike Tanyrhinichthys, Phanerorhynchus lacks a separate jugal and lacrimal (Gardiner, 1967). Instead, Phanerorhynchus has a single infraorbital that occupies the same region as the lacrimal and jugal of Tanyrhinichthys, a feature that is also present in Haplolepidae (Westoll, 1944; Gardiner, 1967; Lowney, 1980).
The post-cranial morphology of Phanerorhynchus is not complete, but is preserved well enough for a detailed comparison. The construction of the fins of Phanerorhynchus differs considerably from Tanyrhinichthys (Gill, 1923; Fig. 16). The lepidotrichia of Phanerorhynchus are thick, few in number and are generally spaced far apart from one another, as in haplolepids (Gill, 1923; Westoll, 1944; Gardiner, 1967; Lowney, 1980). This is different from the thin, closely packed and numerous fin rays of Tanyrhinichthys, aside from the pectoral fin. Additionally, the thick, unjointed lepidotrichia of the anterior portion of the ventral lobe of the caudal fin in Phanerorhynchus are distinct from the corresponding thin, jointed fin rays in the caudal fin of Tanyrhinichthys (Gill, 1923; ‘cf’ in Fig. 16). Finally, the pelvic fin of Phanerorhynchus appears much longer relative to the short and rounded pelvic fin of Tanyrhinichthys (‘pe’ in Fig. 16).
The squamation of Tanyrhinichthys differs from what has been observed in Phanerorhynchus (Gill, 1923; Gardiner, 1967). The dorsal, anal and pelvic fins of Phanerorhynchus are preceded by ridge scales and fulcra that are much larger and more pronounced than those preceding the respective fins of Tanyrhinichthys (Gill, 1923; Gardiner, 1967). The dorsal ridge scales of Phanerorhynchus (‘dr’ in Fig. 16) grade into dorsal basal fulcra that are relatively large spines (Gill, 1923). These differ considerably from the small, un-pointed dorsal basal fulcra in Tanyrhinichthys. Furthermore, the scales of Tanyrhinichthys are much straighter than those of Phanerorhynchus, which have distinctly curved anterior and posterior margins (Gill, 1923). Additionally, the middle flank scales of Phanerorhynchus each bear a tubercle on both their dorsal edges and about two-thirds of the way between their dorsal and posterior edges (Gill, 1923). There is no evidence of such tubercles being present in Tanyrhinichthys.
Tanyrhinichthys is distinctly unlike Phanerorhynchus in the construction of its skull, its fins and its scales. This indicates that Phanerorhynchus and Tanyrhinichthys are separate long-rostrumed lineages that evolved these features due to convergence on a bottom-cruising lifestyle. Phanerorhynchus more closely resembles members of the Haplolepidae in the construction of its skull and fins, particularly haplolepids from the same region of northern England (Lowney, 1980).
Tanyrhinichthys is most similar to the unnamed long-rostrumed actinopterygian from the Logan Quarry of Indiana. Unfortunately, this taxon is known from an isolated and relatively incomplete skull that offers little morphological information for comparison (PF 2289; Poplin, 1978, fig. 1). Additionally, we could not locate PF 2289 at the FMNH (pers. observ.). However, the skull roof of this taxon appears to be similar to that of Tanyrhinichthys in general morphology. In particular, PF 2289 as illustrated by Poplin (1978, fig. 6) closely resembles the skull of NMMNH P-51192 (which is also crushed dorsoventrally). Both of these specimens have narrow skull roofs with long, thin and pointed rostra. Additional material from the Indiana taxon is needed to make a more complete assessment of its relationship to Tanyrhinichthys.
Elongate rostra, broadly defined in actinopterygians as extensions of the bones of the skull or the jaws past the orbit or nares, are extremely common amongst both extinct and extant ray-finned fishes (pers. observ.). Based on our comparisons, elongate rostra evolved at least four separate times amongst Palaeozoic actinopterygians, once in the Devonian (Tegeolepis), at least twice in the Pennsylvanian (Tanyrhinichthys and Phanerorhynchus, potentially a third time in the Indiana taxon) and finally in the Permian (Eosaurichthys). This demonstrates that, although elongate rostra are most common in extant ray-finned fishes (pers. observ.), cranial elongation evolved as early as the Late Devonian and appeared independently in several lineages before the end of the Palaeozoic.
Although each of the long-rostrumed fishes we examined are distinct in rostral structure, there is a broader pattern in cranial elongation. The rostra of Tanyrhinichthys, Phanerorhynchus, the Indiana taxon and Tegeolepis are built primarily from bones of the skull roof and bones associated with the ethmoid region that have been lengthened to produce an elongate snout above the mouth (Fig. 15A). However, the elongate rostrum of Eosaurichthys (like other Saurichthyiformes) is built primarily (not entirely, see discussion above) from elongations of the jaws, giving this fish a lengthened mouth (Fig. 15B). It seems that elongate rostra of at least two distinct forms evolved amongst several lineages of Palaeozoic actinopterygians (Fig. 16). This suggests that lengthened rostra in ray-finned fishes may fall into several distinct general forms or types. A broader survey of long-rostrumed ray-finned fishes that is beyond the scope of this study is required to adequately address this possibility.
Post-Hangenberg convergence and morphological innovation
The Late Pennsylvanian Tanyrhinichthys converged on a sturgeon-like bottom-cruising ecomorphology, representing one of the earliest actinopterygians to exhibit these features. Tanyrhinichthys appeared almost simultaneously with two other bottom-dwelling freshwater forms with elongate rostra, Phanerorhynchus from Lancashire, England, and the elasmobranch chondrichthyan Bandringa (Zangerl, 1969) from Mazon Creek, Illinois, Linton, Ohio, and Cannelton, Pennsylvania (Gardiner, 1967; Sallan & Coates, 2014), both Moscovian in age. Another isolated actinopterygian rostrum comes from similarly aged rocks in Indiana (Poplin, 1978), suggesting the widespread appearance of a then-novel form. In these taxa, the snout is constructed from an extended central element (the rostral bone in the actinopterygians, the cruciform rostral cartilage in Bandringa), supported by paired struts (the nasals in the actinopterygians, selinoid rostral cartilages in Bandringa; Sallan & Coates, 2014). The rostra of these taxa are marked by an increase in the sensory apparatus, as shown by expansion of pores or extension of the lateral line itself towards the distal snout. This excludes Phanerorhynchus, because L. 8585 is not preserved well enough for rostral sensory organs to be present. In Tanyrhinichthys and Bandringa, the flattened rostral extension (like that of the unnamed Indiana actinopterygian) is separated from the gape by a rounded element surrounding a fenestra of unknown purpose, which may have contained additional sensory tissues (as above; Sallan & Coates, 2014, fig. 4; Figs 3A, B, 4).
The degree of convergence in these distantly related, long-rostrumed fishes is remarkable, especially considering the novelty of their ecomorphologies in the Carboniferous and their overlapping or near-overlapping age estimates in the Late Pennsylvanian. The timing may not be a coincidence given that Phanerorhynchus, Bandringa and the Indiana taxon are found in coal measures, including river settings containing abundant plant matter (Gill, 1923; Gardiner, 1967; Poplin, 1978; Sallan & Coates, 2014). The murky bottom waters of Carboniferous river systems, choked with decaying, carbon-rich leaves, may have provided abundant food while presenting challenges for visual hunting (Baird, 1997; Sallan & Coates, 2013). While Tanyrhinichthys is not found in coal deposits, its rarity at KBQ suggests that it may have come from such, much as Bandringa is also found in nearshore marine settings at Mazon Creek (Sallan & Coates, 2014). Alternatively, the estuarine setting of KBQ may have generated enough sediment to also favour fewer visual modes of prey detection. These novel environments and the challenges they presented to visual hunting may have forced the evolution of snout-based detection systems, as has been hypothesized for both the American paddlefish Polyodon spathula (Walbaum, 1792) and sturgeon (Harkness & Dymond, 1961; Jørgensen et al., 1972; Grande & Bemis, 1991; Wilkens et al., 1997; Peterson et al., 2007). These modern analogues suggest that the simultaneous appearance of elongate rostra bearing with sensory organs amongst Carboniferous fishes was driven by the challenges to visual predation that arose in novel environments.
The long-rostrumed Pennsylvanian fishes are one example of a repeated pattern of convergent innovation within the diversification of vertebrates following the end-Devonian Hangenberg event (359 Mya; Sallan & Coates, 2010; Sallan, 2014). There are several other simultaneous or near simultaneous first appearances of ecotypes among both actinopterygians and chondrichthyans (especially holocephalans) during the Carboniferous, sometimes in the same ecosystem (Sallan & Coates, 2010, 2013; Sallan et al., 2011; Sallan, 2012). One example is the Mississippian origination of deep-bodied, laterally flattened ‘reef’ fishes among multiple lineages of actinopterygians, e.g. Eurynotiformes such as Cheirodopsis (Traquair, 1881) and platysomids such as Platysomus (Traquair, 1881) from the Visean of Glencartholm, Scotland, Frederichthys (Coates, 1993) from the Serpukhovian of Bearsden, Scotland, Proceramala (Poplin & Lund, 2000) and Discoserra (Lund, 2000) from the Serpukhovian of Bear Gulch, Montana and Adroichthys (Gardiner, 1969) from the Visean of South Africa (Traquair, 1881: Moy-Thomas & Bradley Dyne, 1938; Gardiner, 1969; Coates, 1993; Lund, 2000; Poplin & Lund, 2000; Hurley et al., 2007; Sallan & Coates, 2013). Examples of deep-bodied forms also occur among chondrichthyans [the petalodont Belantsea (Lund, 1989) and Echinochimaera (Lund, 1977)] and even coelacanths [Allenypterus (Lund & Lund, 1984)] from Bear Gulch (Lund, 1977, 1989; Lund & Lund, 1984). Nearly all of these fishes are durophages, presaging later, deep-bodied stem-teleost pycnodonts and modern teleost durophages (sparids, wrasses, parrotfish and tetraodoniforms) and are coincident with a large number of durophagous chondrichthyans, lungfishes and actinopterygians with other body types (Bellwood, 2004; Sallan et al., 2011; Sallan & Coates, 2013).
Another example of convergent innovation in Carboniferous fishes is axially elongated ‘eels’ with reduced paired fins and continuous median-caudal fins. This body form has been observed in actinopterygians from Glencartholm [Tarrasius (Traquair, 1881)] and Bear Gulch [Paratarrasius (Lund & Melton, 1982; Sallan, 2012)], chondrenchelyid holocephalans from Glencartholm [Chondrenchelys (Traquair, 1888)] and Bear Gulch (Harpagofututor; Lund, 1982; Lund & Melton, 1982; Finarelli & Coates, 2014), elasmobranch chondrichthyans from the Permian of Europe and North America [Orthacanthus (Agassiz, 1843)] and Bear Gulch (Thrinacoselache; Zangerl, 1981; Grogan & Lund, 2008; Sallan, 2012) and possibly coelacanths (‘Apholidotos’, an undescribed but named taxon previously attributed to the polyphyletic actinopterygian family ‘tarrasiidae’ but excluded by more recent work; LS pers. observ.; Frickhinger, 1991; Lund & Poplin, 2002; Sallan, 2012).
The convergent taxa mentioned above, including Tanyrhinichthys, are only the most extreme and noticeable examples of duplicated, coincident innovations among Late Palaeozoic fishes (Fig. 17). The repeated appearance of convergent forms suggests that shared environmental pressures and functional demands existed across Carboniferous marine and freshwater ecosystems. The novel morphologies that first occur among multiple Carboniferous lineages mirror the morphological diversity of later fish clades, such as neopterygians and teleosts in the Mesozoic and Cenozoic (Sallan & Friedman, 2012). However, chondrichthyans after the Palaeozoic seem to have become incapable of generating some of the more specialized ‘reef’ forms, such as eels and deep-bodied ‘angelfish,’ in line with a dramatic loss in relative holocephalan diversity and richness (Friedman & Sallan, 2012).
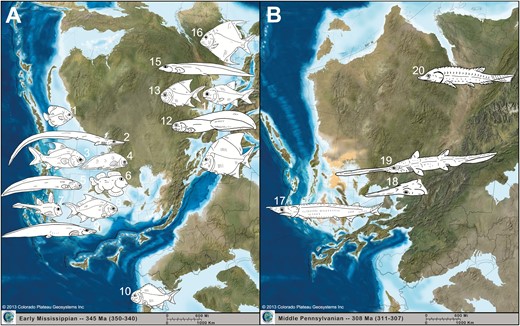
Convergent morphological innovation in post-Hangenberg fishes. A, occurrence of deep-bodied and eel-like actinopterygians, chondrichthyans and coelacanths in the Mississippian: 1, Discoserra; 2, Thrinacoselache; 3, Aesopichthys; 4, Allenypterus; 5, Paratarrasius; 6, Belantsea; 7, Echinochimaera; 8, Proceramala; 9, Harpagofututor; 10, Adroichthys; 11, Platysomus; 12, Tarrasius; 13, Paramesolepis; 14, Frederichthys; 15, Chondrenchelys; 16, Amphicentrum. B, occurrence of long-rostrumed actinopterygians and chondrichthyans in the Pennsylvanian: 17, Tanyrhinichthys; 18, undescribed long-rostrumed taxon from Logan Quarry, Indiana; 19, Bandringa; 20, Phanerorhynchus. Fishes not to scale. Maps (Key Time Slices of North America, 308 MA and 345 MA) were created by Ron Blakey at Colorado Plateau Geosystems Inc., used under License #61019, ©2013 Colorado Plateau Geosystems Inc.
A global shift in the relative evolvability and viability of fish ecotypes seems to have occurred in the Carboniferous. This was perhaps contingent on the new dominance of actinopterygians and chondrichthyans after the end-Devonian extinction and/or a coincident change in the basic structures of aquatic vertebrate ecosystems or their environments (Sallan & Galimberti, 2015). This new state of fish faunas appears to have lasted to the present day, even as one of the two dominant groups, chondrichthyans, stopped producing the more extreme forms.
CONCLUSIONS
Our revision of the morphology of Tanyrhinichthys indicates that it was most likely a bottom-cruising predator similar in general ecomorphology to modern sturgeon, as these taxa share a set of features associated with a benthic lifestyle. Our examination of Tanyrhinichthys and broadly contemporaneous long-rostrumed ray-finned fishes demonstrates that elongate rostra evolved independently in several lineages of Palaeozoic actinopterygians, as well as at least one chondrichthyan. The bottom-cruising ecomorphology of Tanyrhinichthys evolved within the context of widespread, often simultaneous and coincident convergence on then-novel ecomorphologies amongst disparate lineages of actinopterygians and chondrichthyans in the wake of the end-Devonian Hangenberg extinction, a phenomenon that appears to have extended into the Late Pennsylvanian and until today.
ACKNOWLEDGEMENTS
We thank Amanda Cantrell and Tom Suazo (NMMNH) and Amy Henrici (CMNH) for their help in the collections, lab and field. We also thank Emma Bernard (NHM), Hans-Peter Schultze (KUVP), William Simpson (FMNH) and Adrienne Stroup (FMNH) for their assistance and providing access to crucial specimens. We thank MattFriedman (U. Michigan) for providing useful scans. This study includes data produced in the CTEES facility at University of Michigan, supported by the Department of Earth & Environmental Sciences and College of Literature, Science & the Arts. We also thank the reviewers for their constructive comments. Finally, we thank John Sime and Maisie O’Brien for their help in the drawings required for this manuscript.
FUNDING
This work was supported by the University of Pennsylvania Paleontology Summer Stipend (to Jack Stack), the Greg and Susan Walker Endowment for Student Research in Earth & Environmental Science (to Jack Stack), the Paleontological Society Student Ambassador Award (to Jack Stack), the University of Pennsylvania Grant for Faculty Mentoring Undergraduate Research (to Lauren Sallan) and NSF CAREER 1846777 (to Lauren Sallan).
REFERENCES